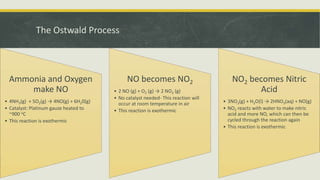
The Ostwald process is a 3-step reaction that oxidizes ammonia to produce nitric acid in a more economical way than directly reacting nitrogen with oxygen. Wilhelm Ostwald patented the process in 1902. It involves reacting ammonia with oxygen over a platinum catalyst to produce NO, then reacting the NO with oxygen to produce NO2, and finally reacting the NO2 with water to produce nitric acid. The process allows for cheap, mass production of nitric acid which is used to produce ammonium nitrate fertilizer and explosives like nitroglycerin and trinitrotoluene.