The document covers the properties and reactions of nitrogen and its compounds, focusing on the manufacture of ammonia via the Haber process and nitric acid via the Ostwald process. It details the chemical properties, applications, and harmful effects of ammonia, as well as the electronic configuration and inertness of nitrogen. Additionally, it highlights the various oxyacids of nitrogen and the acid and oxidizing nature of nitric acid in chemical reactions.

![IR CHEM 2
Course Content
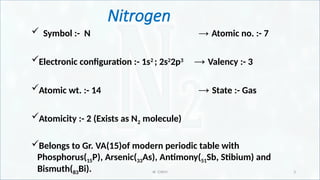
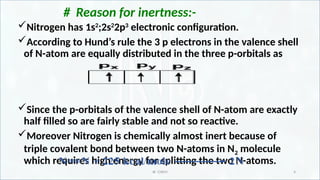
Reason for inertness of nitrogen and active nitrogen
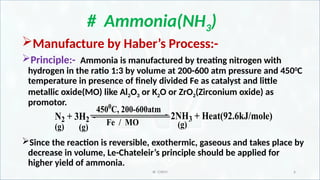
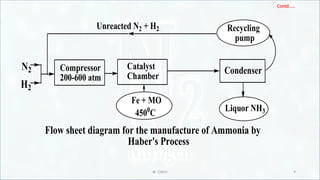
Manufacture of Ammonia by Haber’s process
Chemical properties of ammonia [Action with water, Conc. HCl, O2,
CuSO4 solution, FeCl3 solution, Mercurous nitrate paper ]
Applications of ammonia, Harmful effects of ammonia
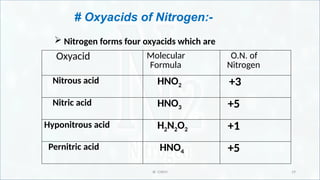
Oxy-acids of nitrogen (name and formula)
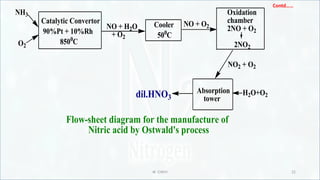
Manufacture of nitric acid by Ostwald's process
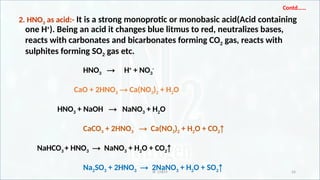
Chemical properties of nitric acid [HNO3 as an acid and oxidizing agent
(action with zinc, magnesium, iron, copper, sulphur, carbon, SO2 and
H2S)
Ring test for nitrate ion.](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-2-320.jpg)
















![IR CHEM 27
Contd…...
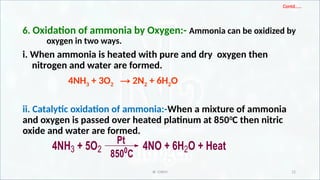
a. Oxidation of Zinc:-
i. With conc.HNO3:- Zinc nitrate, water and NO2 gas are formed.
ii. With 1:1 HNO3:- Zinc nitrate, water and NO gas are formed.
Zn + 2HNO3 Zn(NO3)2 +2[H]
HNO3 + [H] H2O + NO2 ] x 2
Zn + 4HNO3 Zn(NO3)2 + 2H2O + 2NO2](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-27-320.jpg)
![IR CHEM 28
Contd…...
iii. With dil. HNO3:- Zinc nitrate, water and N2O gas are formed.
iv. With very dil.HNO3:- Zinc nitrate, ammonium nitrate and water
are formed.
Zn + 2HNO3 Zn(NO3)2 + 2[H] ] x 4
2HNO3 +8[H] 5H2O + N2O
4Zn + 10HNO3 4Zn(NO3)2 + 5H2O + N2O](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-28-320.jpg)
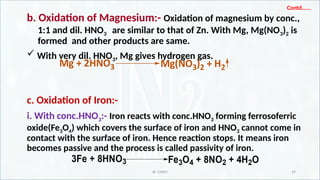
![IR CHEM 30
Contd…...
ii. With 1:1 HNO3:- Iron reacts with 1:1 HNO3 forming ferric nitrate,
NO2 gas and water.
iii. With dil. HNO3:- Iron reacts with dil HNO3 forming ferrous nitrate,
N2O gas and water.
Fe+ 3HNO3 Fe(NO3)3 +3[H]
HNO3 + [H] H2O + NO2 ] x 3
Fe + 6HNO3 Fe(NO3)3 + 3H2O + 3NO2
Fe+ 2HNO3 Fe(NO3)2 +2[H] x 4
2HNO3 + 8[H] 5H2O + N2O
4Fe + 10HNO3 4Fe(NO3)2 + 5H2O + N2O](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-30-320.jpg)
![IR CHEM 31
Contd…...
iv. With very dil.HNO3:- Iron reacts with very dil HNO3 forming ferrous
nitrate, ammonium nitrate and water.
Y. Oxidation of metals lying below Hydrogen in the
electrochemical series:-
Only conc. and 1:1 HNO3 can oxidize the metals lying below
hydrogen in the electrochemical series.
Fe + 2HNO3 Fe(NO3)2 + 2[H] ] x 4
HNO3 +8[H] 3H2O + NH3
4Fe + 10HNO3 4Fe(NO3)2 +
NH3 + HNO3 NH4NO3
NH4NO3+ 3H2O](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-31-320.jpg)

![IR CHEM 33
a. Oxidation of Copper
i. With conc. HNO3:- Cupric nitrate, water and NO2 gas are formed.
ii. With 1:1 HNO3:- Cupric nitrate, water and NO gas are formed.
H2O + 2NO2 + [O]
Cu + [O] CuO
CuO + 2HNO3 Cu(NO3)2 + H2O
Cu + 4HNO3 Cu(NO3)2 + 2H2O + 2NO2
2HNO3
H2O + 2NO + 3[O]
Cu + [O] CuO] x 3
CuO + 2HNO3 Cu(NO3)2 + H2O ]x 3
3Cu + 8HNO3 3Cu(NO3)2 + 4H2O + 2NO
2HNO3](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-33-320.jpg)

![IR CHEM 35
Contd…...
a. Oxidation of Carbon:- Carbon is oxidised to carbon dioxide by
conc. HNO3.
b. Oxidation of Sulphur:- Sulphur is oxidised to sulphuric acid by
conc. HNO3.
2HNO3 H2O + 2NO2 + [O] ]x 2
C + 2[O] CO2
C + 4HNO3 2H2O + 4NO2 + CO2
2HNO3 H2O + 2NO2 + [O] ]x 3
S + 3[O] SO3
S + 6HNO3 H2SO4 + 2H2O + 6NO2
SO3 + H2O H2SO4](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-35-320.jpg)

![IR CHEM 37
Contd…...
a. Oxidation of hydrogen sulphide(H2S):- H2S is oxidised to sulphur
by conc.HNO3.
b. Oxidation of Sulphur dioxide(SO2):- SO2 is oxidised to sulphuric
acid by conc.HNO3.
2HNO3 H2O + 2NO2 + [O]
H2S + [O] H2O + S
H2S + 2HNO3 2H2O + 2NO2 + S
2HNO3 H2O + 2NO2 + [O]
SO2 + H2O + [O]
SO2 + 2HNO3 H2SO4 + 2NO2
H2SO4](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-37-320.jpg)
![IR CHEM 38
Contd.…..
c. Oxidation of Ferrous sulphate(FeSO4):- FeSO4 is oxidised to
ferric sulphate by conc.HNO3 in presence of dil. H2SO4.
2HNO3 H2O + 2NO2 + [O]
2FeSO4 + H2SO4 + [O]
2FeSO4 + 2HNO3 + H2SO4
Fe2(SO4)3 + H2O
Fe2(SO4)3 + 2H2O + 2NO2](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-38-320.jpg)

![IR CHEM 40
Contd…..
NO3
-
+ H2SO4 → HNO3 + HSO4
-
2HNO3 → H2O + 2NO + 3[O]
2FeSO4 + H2SO4 + [O] → Fe2(SO4)3 + H2O
FeSO4 +5H2O + NO → [Fe(H2O)5NO]SO4
(Pentaaquanitrosyl iron(II)sulphate)
(Brown ring)
# Uses:-
1. Widely used in the manufacture of chemical fertilizers like
ammonium nitrate, calcium ammonium nitrate(CAN) etc.
2. Used in the manufacture of artificial silk.](https://image.slidesharecdn.com/3nitrogen-250130032647-8e4fc6d8/85/3-Nitrogen-pptxsssssssssddddddddddddddddd-40-320.jpg)


