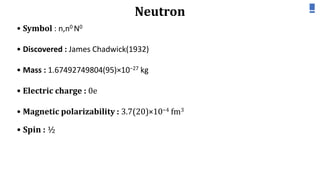
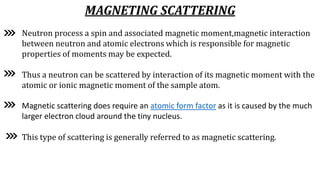
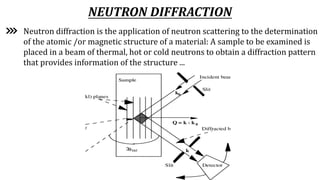
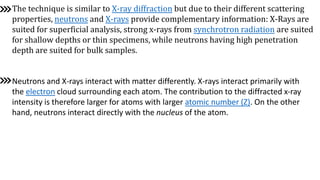
Neutron diffraction uses neutron scattering to determine the atomic and magnetic structure of materials. Neutrons interact with atomic nuclei through nuclear forces and with magnetic moments through their own magnetic moment. This allows neutron diffraction to probe both atomic structure and magnetic structure. It has advantages over x-ray diffraction as neutrons can penetrate bulk samples and are sensitive to lighter elements. Neutron diffraction is widely used to study crystal and magnetic structures.