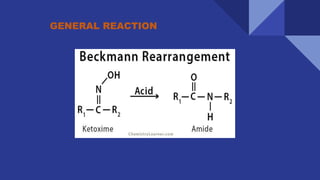
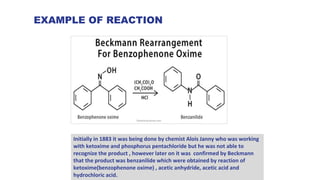
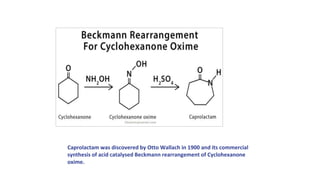
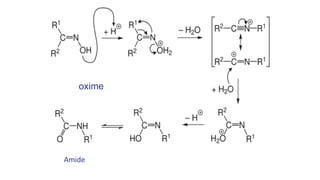
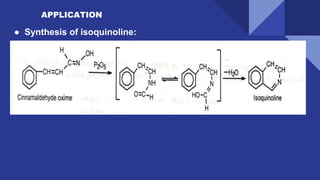
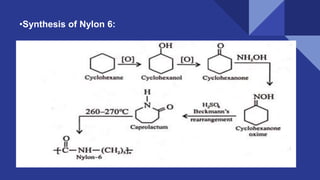
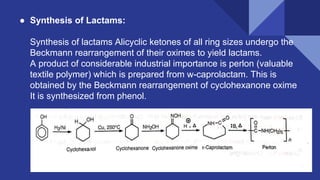
The document discusses the Beckmann rearrangement reaction, which involves the acid-catalyzed conversion of oximes to amides. This reaction allows for the insertion of a nitrogen atom from a C=N double bond into a carbon chain to form a C-N single bond. Concentrated sulfuric acid and other acids can be used as reagents. The migration of groups in this reaction depends on the orientation relative to the -OH group, in an anti position. Examples are given of industrial uses of this reaction in synthesizing isoquinoline, nylon 6, and lactams like caprolactam. The mechanism involves protonation of the oxime, migration of an alkyl group to the nitrogen, and hydrolysis of the