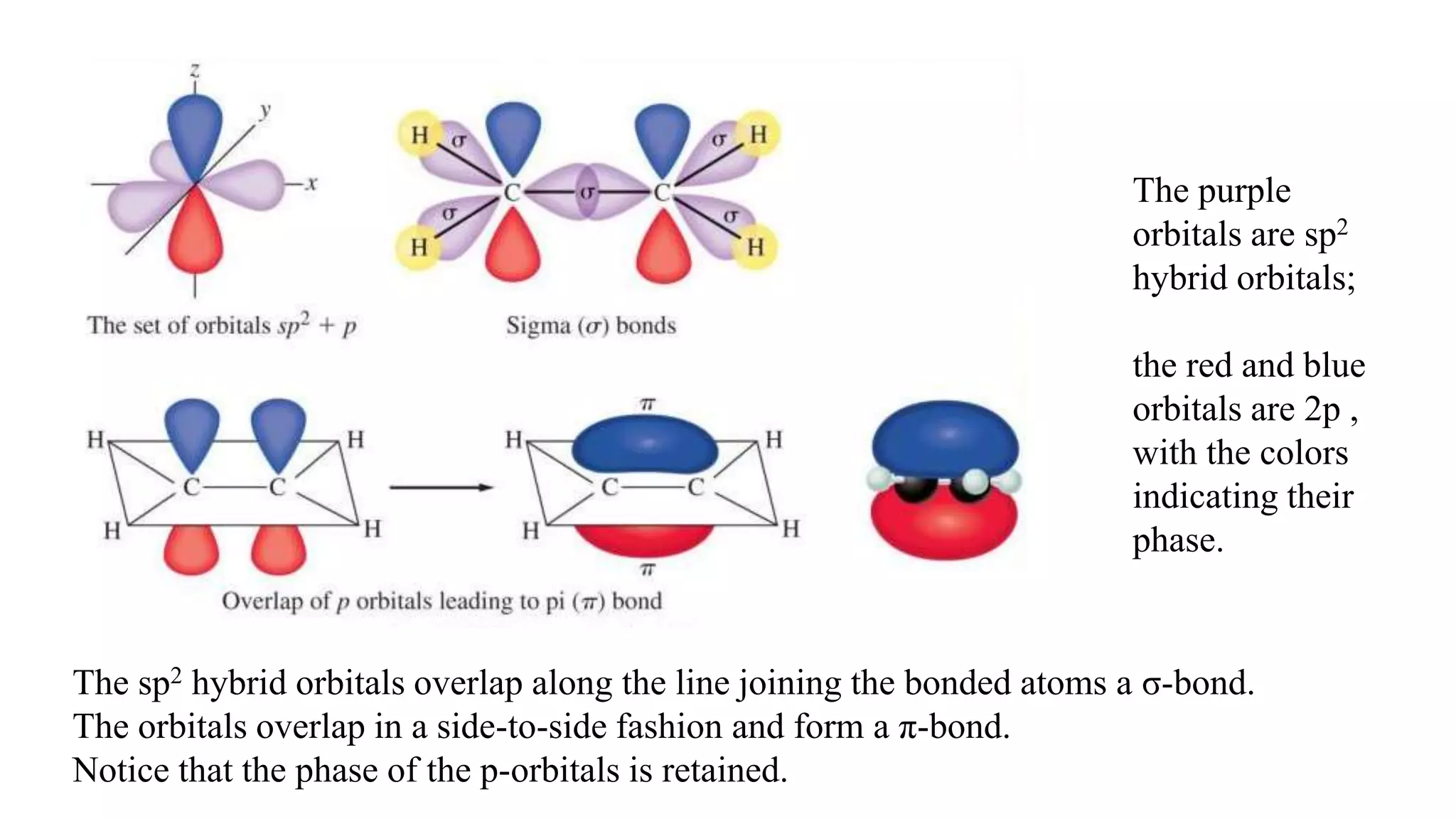
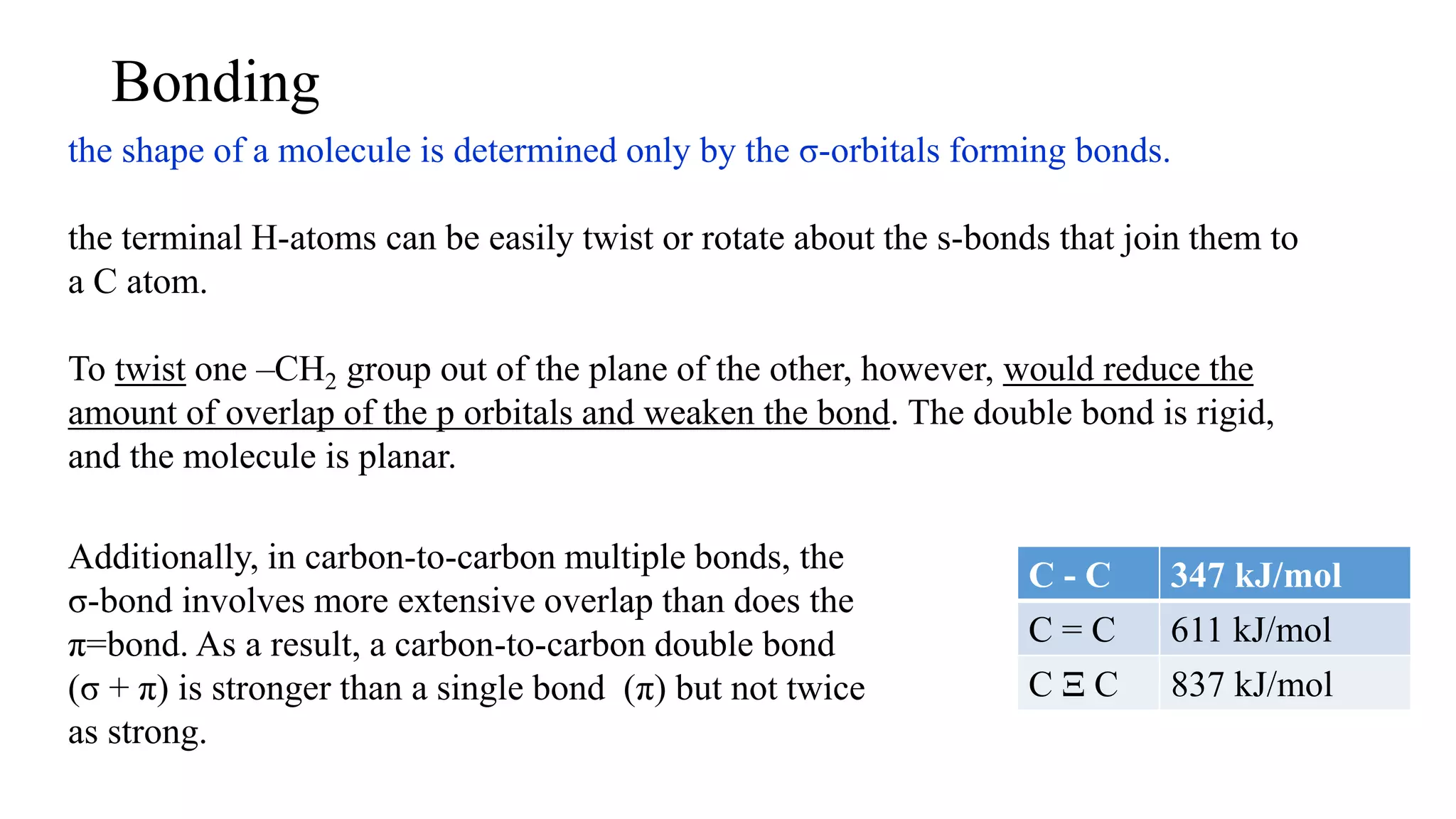
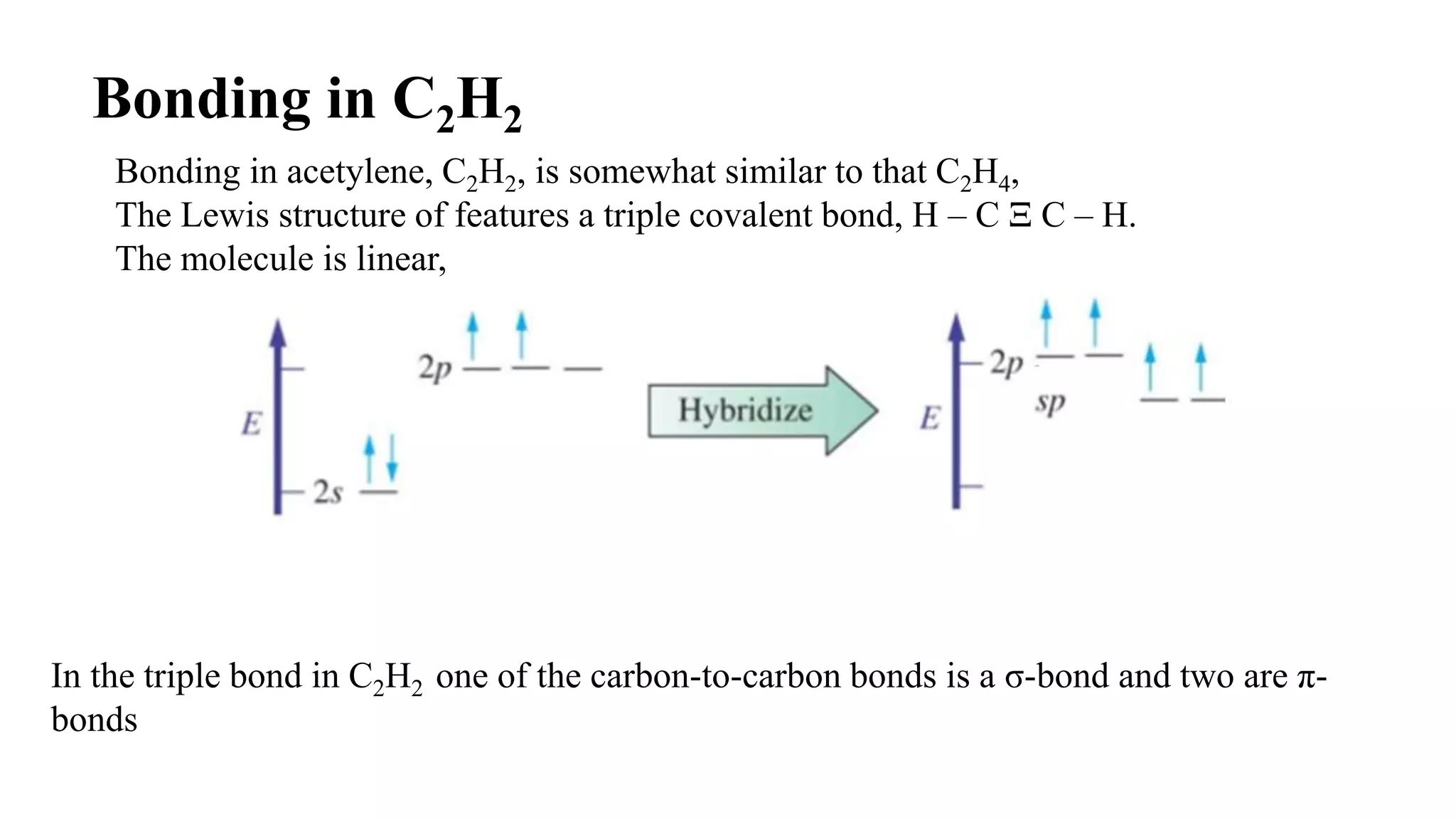
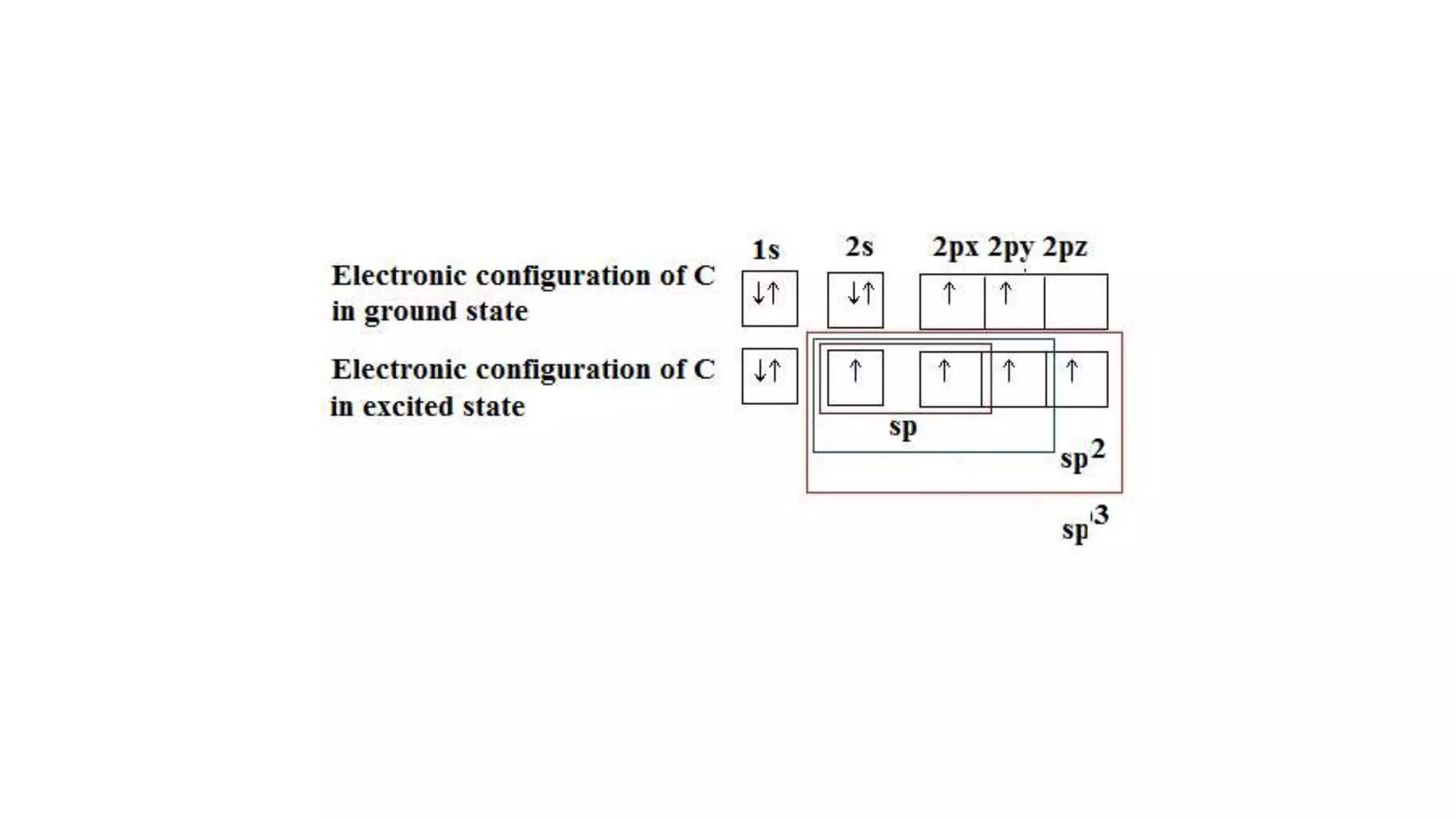
The document discusses bonding in hydrocarbons, specifically alkanes, alkenes, and alkynes, highlighting the hybridization of carbon atoms and the types of bonds formed. It explains how ethylene (C2H4) features a carbon-to-carbon double bond with sp2 hybridization, resulting in a planar structure, while acetylene (C2H2) has a linear arrangement due to its triple bond. The strength and nature of sigma (σ) and pi (π) bonds are also compared, noting that σ bonds are stronger due to more extensive overlap.