Carbon is the fourth most abundant element in the universe, forming a vast number of compounds primarily known as organic compounds due to their origin from living sources. Covalent bonds, which can be single, double, or triple, are responsible for the formation of carbon compounds and affect their physical properties, such as low melting and boiling points. Additionally, carbon's ability to catenate enables the formation of various hydrocarbons, including alkanes, alkenes, and alkynes, and they are categorized based on their bonding characteristics and functional groups.










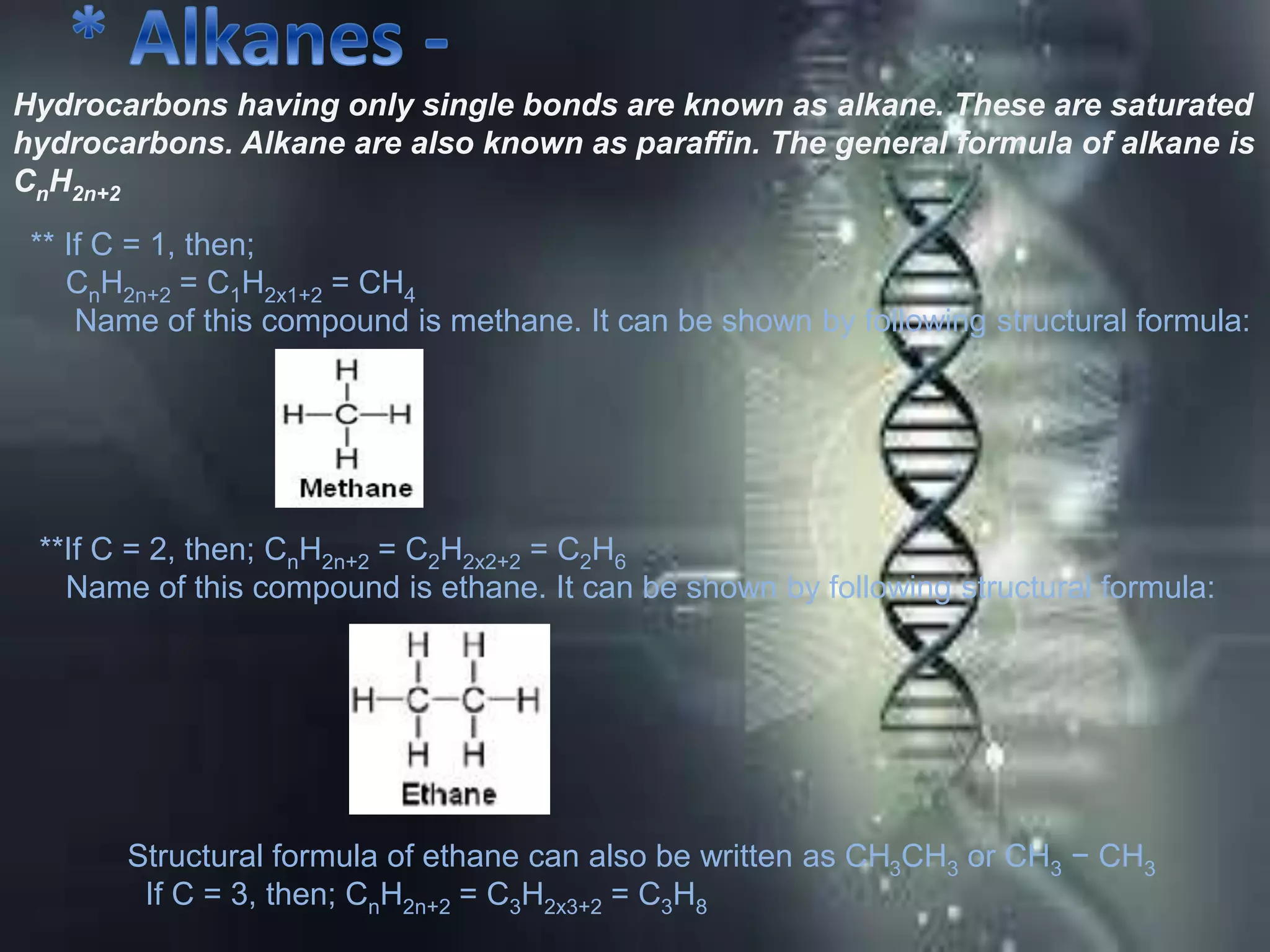
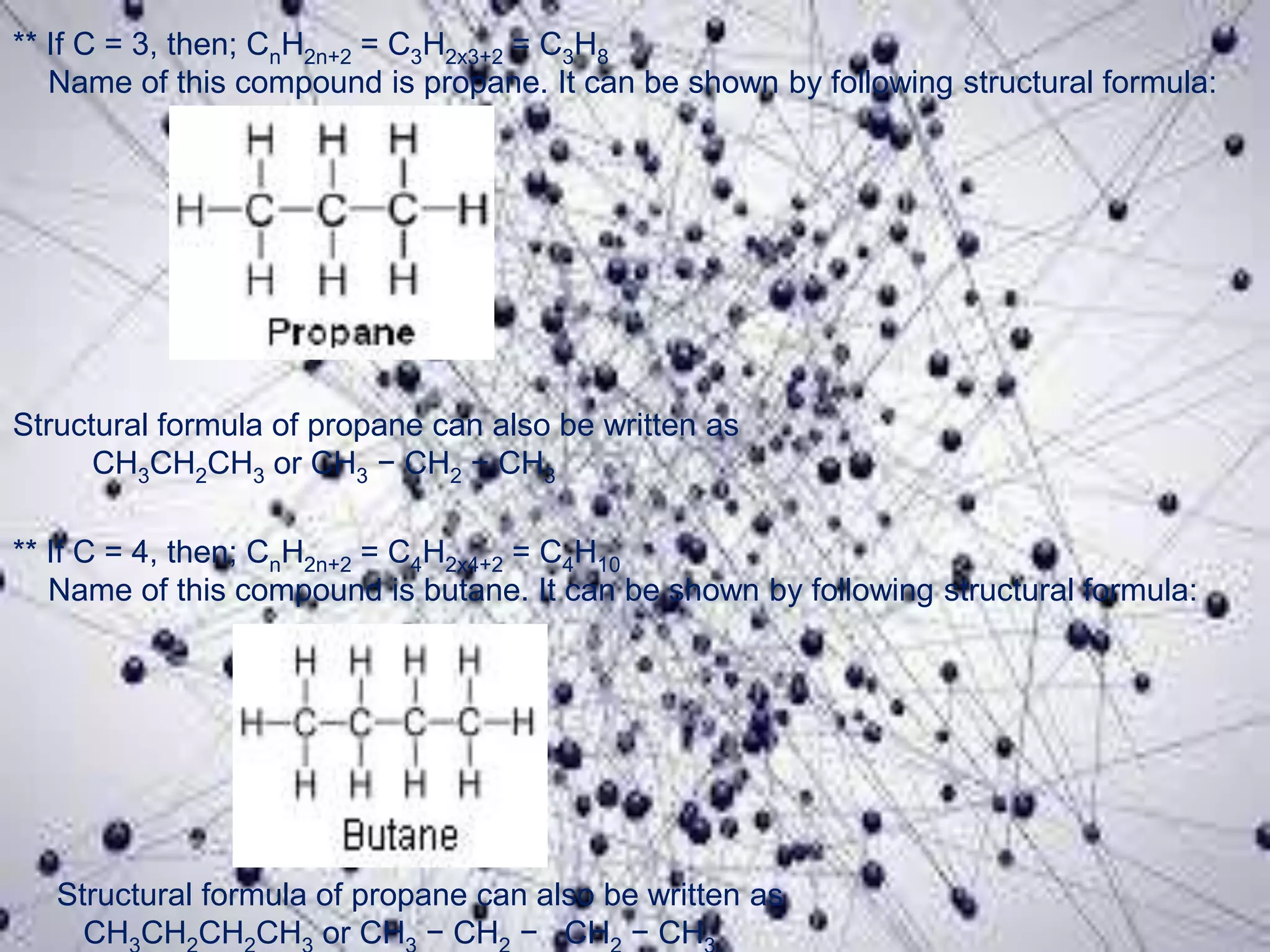
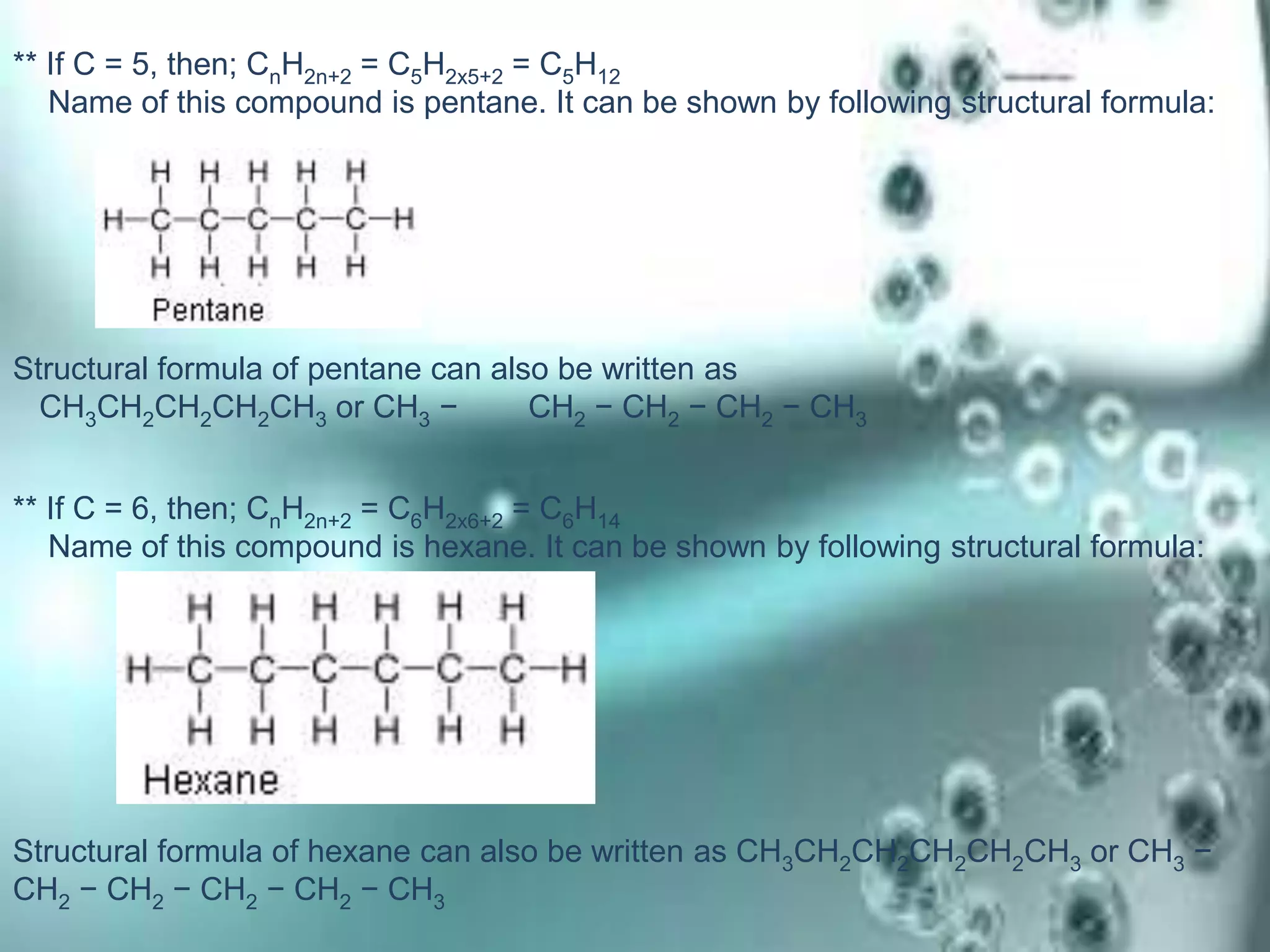





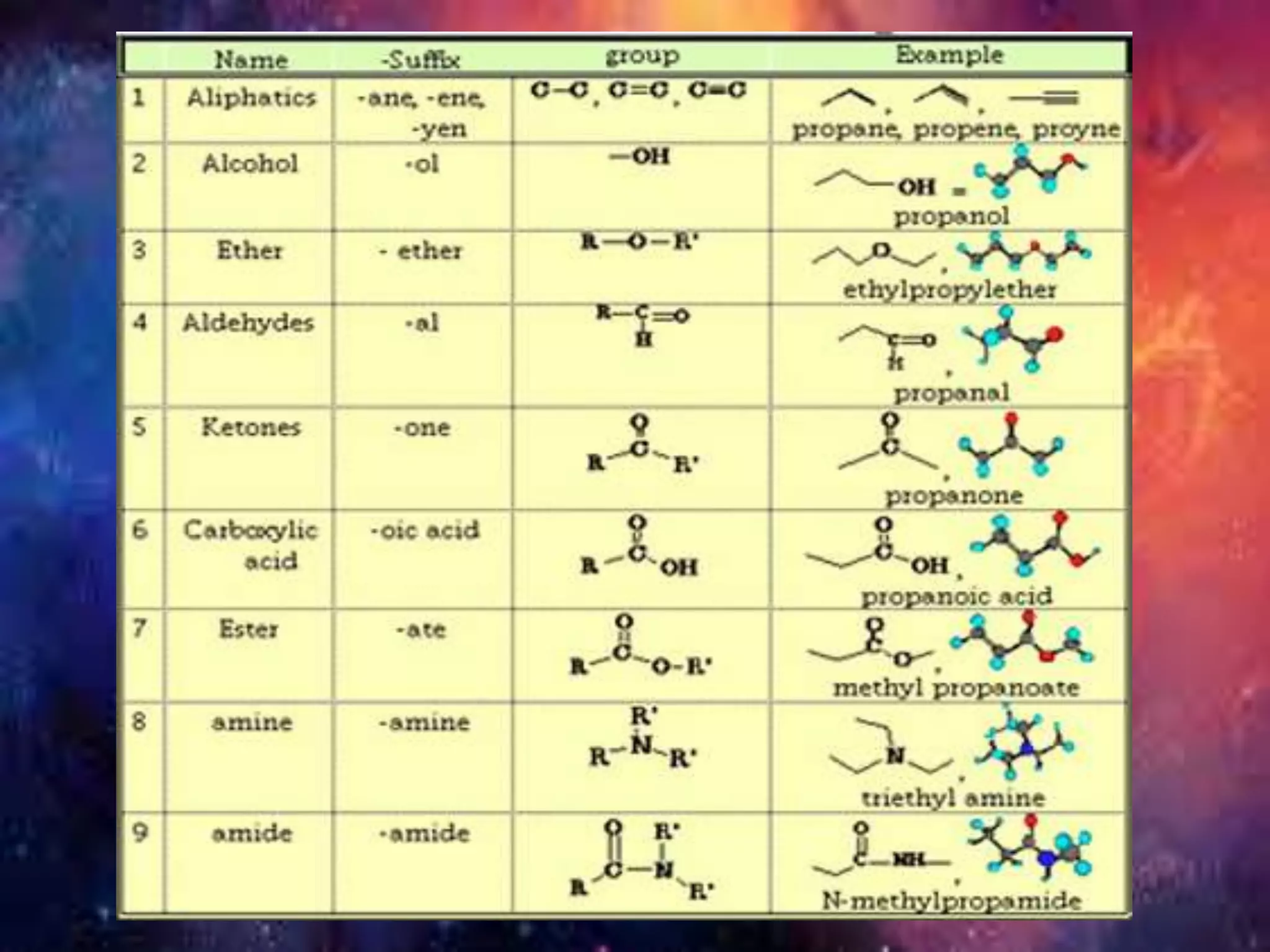

![Carbon and Its compounds. [ Class- X ]](https://image.slidesharecdn.com/scienceproject-160112145158/75/Carbon-and-Its-compounds-Class-X-21-2048.jpg)