The document discusses several key concepts in chemistry:
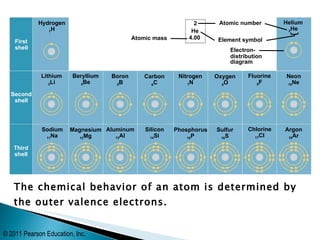
- An atom is the smallest unit of matter that retains the properties of an element. It is determined by its outer valence electrons.
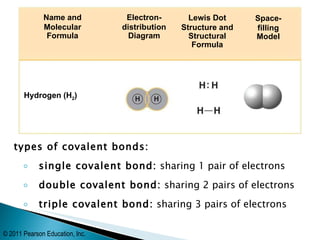
- There are three main types of covalent bonds: single (sharing 1 pair of electrons), double (sharing 2 pairs), and triple (sharing 3 pairs).
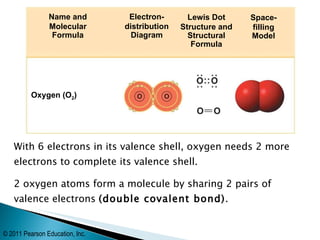
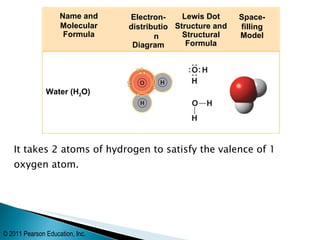
- Oxygen forms a double covalent bond in its O2 molecule by sharing two pairs of valence electrons between two oxygen atoms. Water is formed when two hydrogen atoms share their single electron with oxygen's incomplete octet.
![atom: smallest unit of matter that still retains the properties of an element By Fastfission at en.wikipedia [CC-BY-SA-3.0 (www.creativecommons.org/licenses/by-sa/3.0/), GFDL (www.gnu.org/copyleft/fdl.html) or GFDL (www.gnu.org/copyleft/fdl.html)], from Wikimedia Commons](https://image.slidesharecdn.com/bio106covalentbonds-111029120021-phpapp02/85/BIO106-Covalent-Bonds-1-320.jpg)