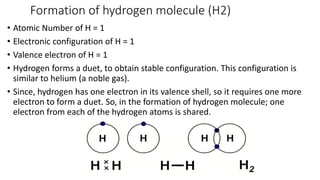
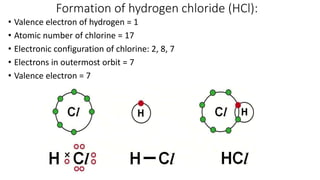
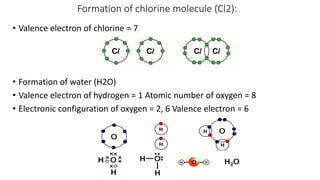
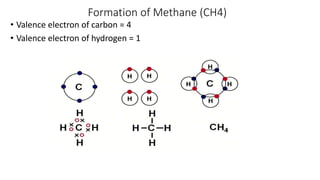
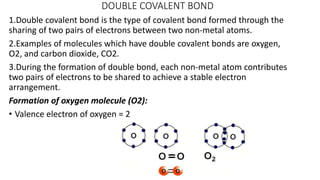
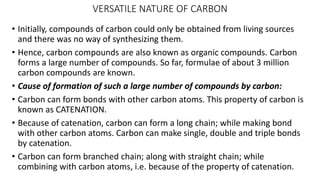
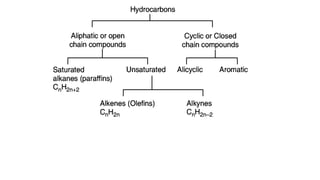
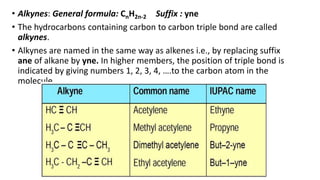
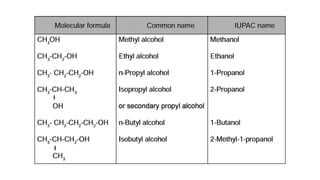
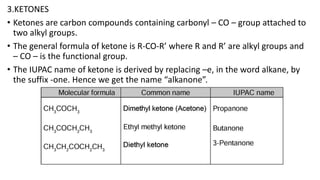
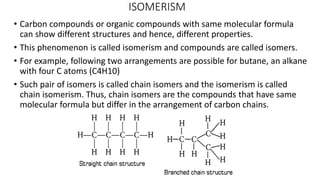
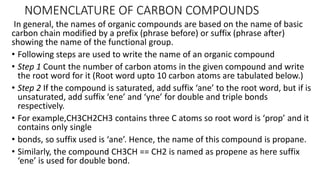
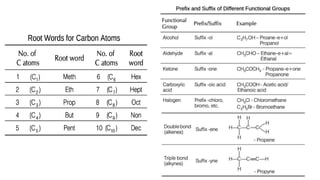
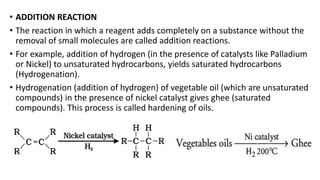
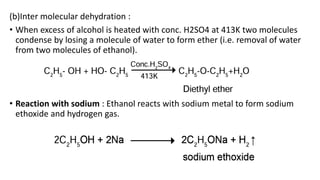
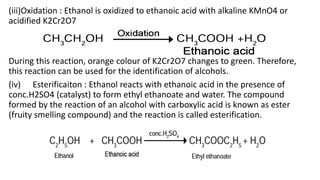
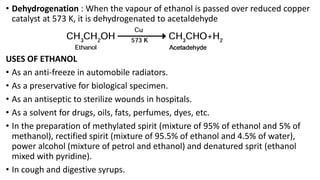
Carbon is the fourth most abundant element in the universe and second most abundant in the human body. It exists in several allotropes including diamond, graphite, and fullerenes. Carbon forms covalent bonds by sharing electrons in stable octets. It exhibits catenation, allowing it to form single, double, and triple bonds with other carbon atoms to create a huge variety of organic compounds. The key classes of organic compounds are hydrocarbons including alkanes, alkenes, and alkynes, as well as functional groups including alcohols, aldehydes, ketones, and carboxylic acids. Isomerism allows different structural isomers of the same molecular formula.