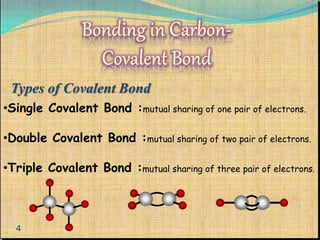
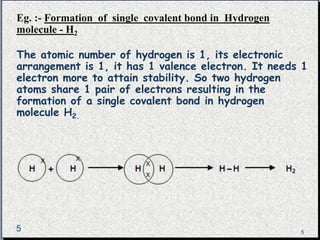
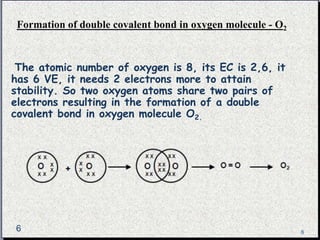
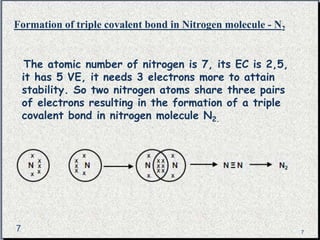
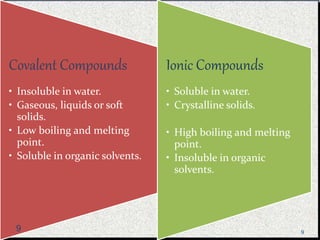
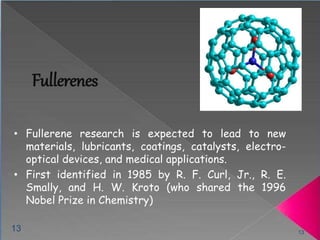
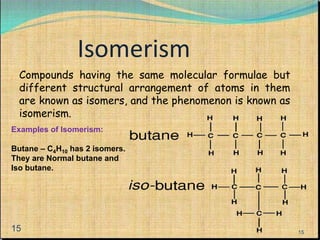
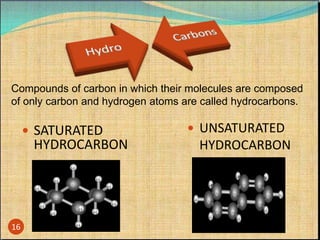
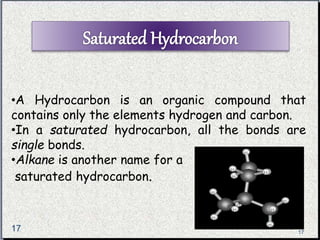
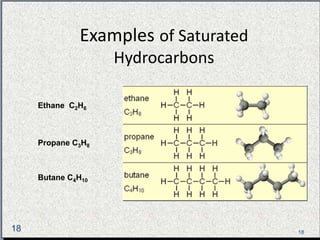
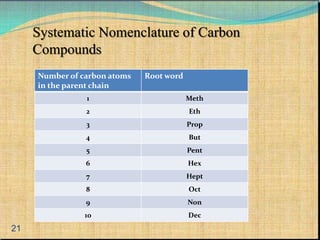
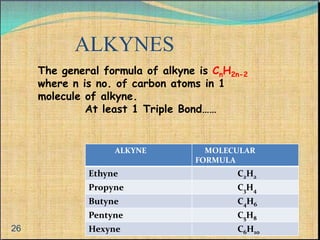
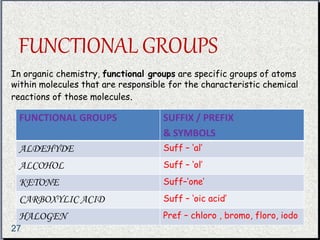
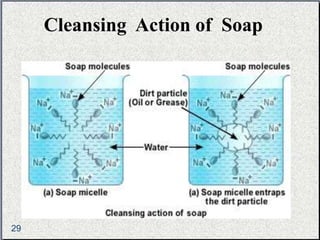
The document provides a comprehensive overview of carbon, its chemical structure, properties, and the types of covalent bonds formed, including examples with hydrogen, oxygen, and nitrogen. It also covers concepts in organic chemistry, such as hydrocarbons, isomerism, and functional groups, alongside their significance and differentiation between soaps and detergents. Additionally, it discusses carbon allotropes like graphite and diamond, and emphasizes the importance of understanding carbon compounds in various scientific applications.