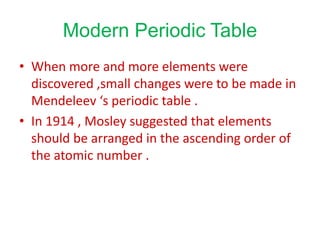
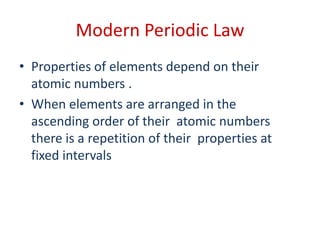
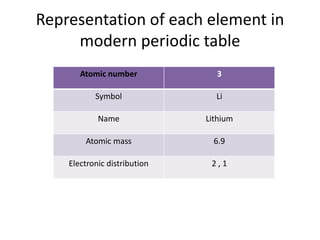
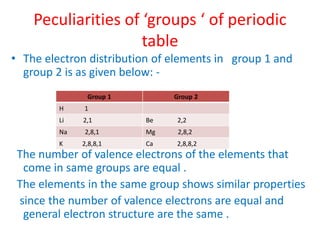
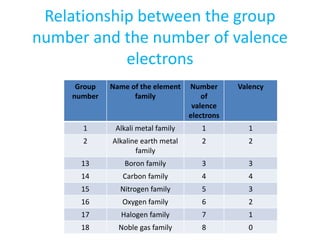
The document discusses the modern periodic table and atomic structure. It describes how Henry Moseley's studies in the early 1900s proved that an element's properties depend on its atomic number rather than atomic mass. The periodic table is now arranged based on increasing atomic number. There are 7 periods and 18 groups in the periodic table. The number of valence electrons corresponds to the group number for groups 1 and 2. Transition elements are those in groups 3 through 12 and have properties between metals and non-metals.