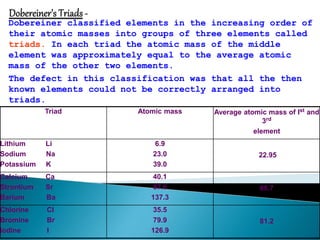
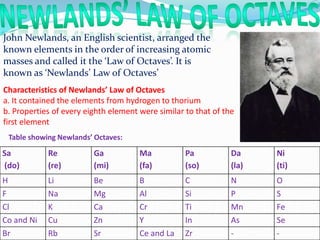
1) The document discusses the periodic classification of elements, including early attempts by Dobereiner, Newlands, and Mendeleev.
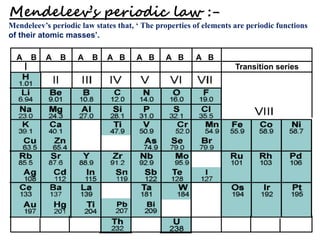
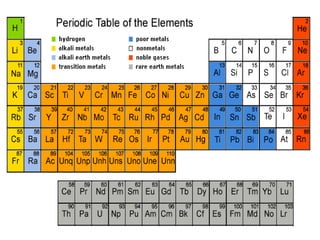
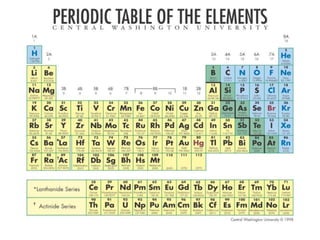
2) Mendeleev organized the elements into a periodic table based on atomic mass and properties, leaving gaps for undiscovered elements.
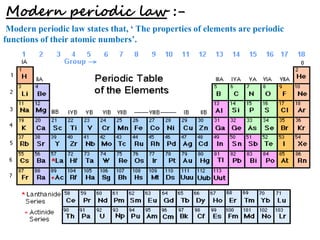
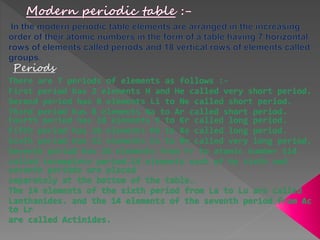
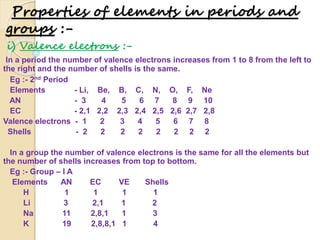
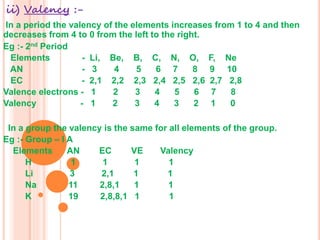
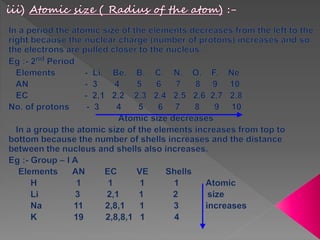
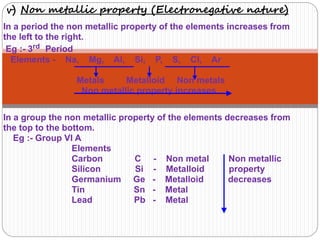
3) Modern periodic law states that properties are a periodic function of atomic number, with elements in the same group having the same number of valence electrons.