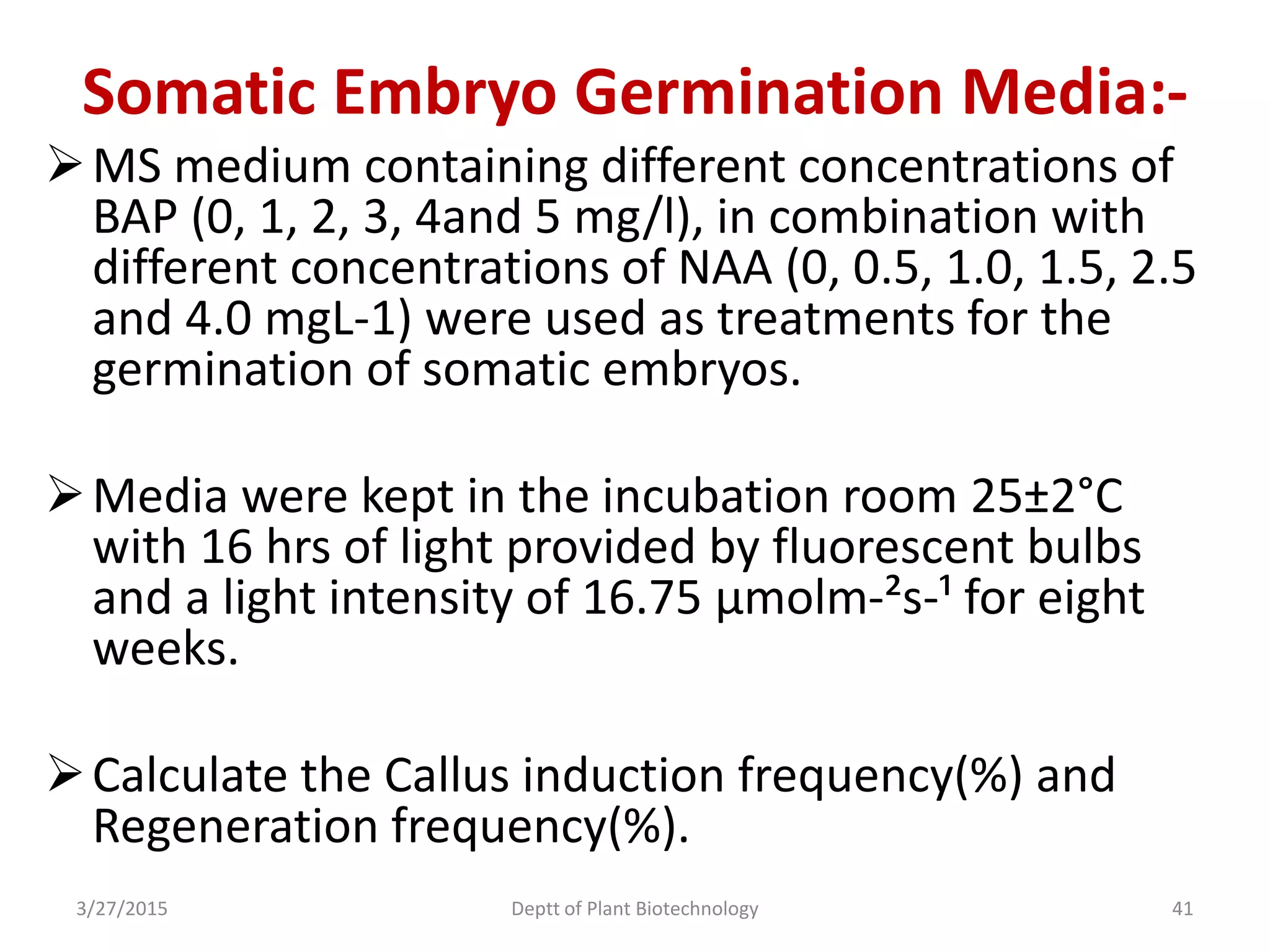
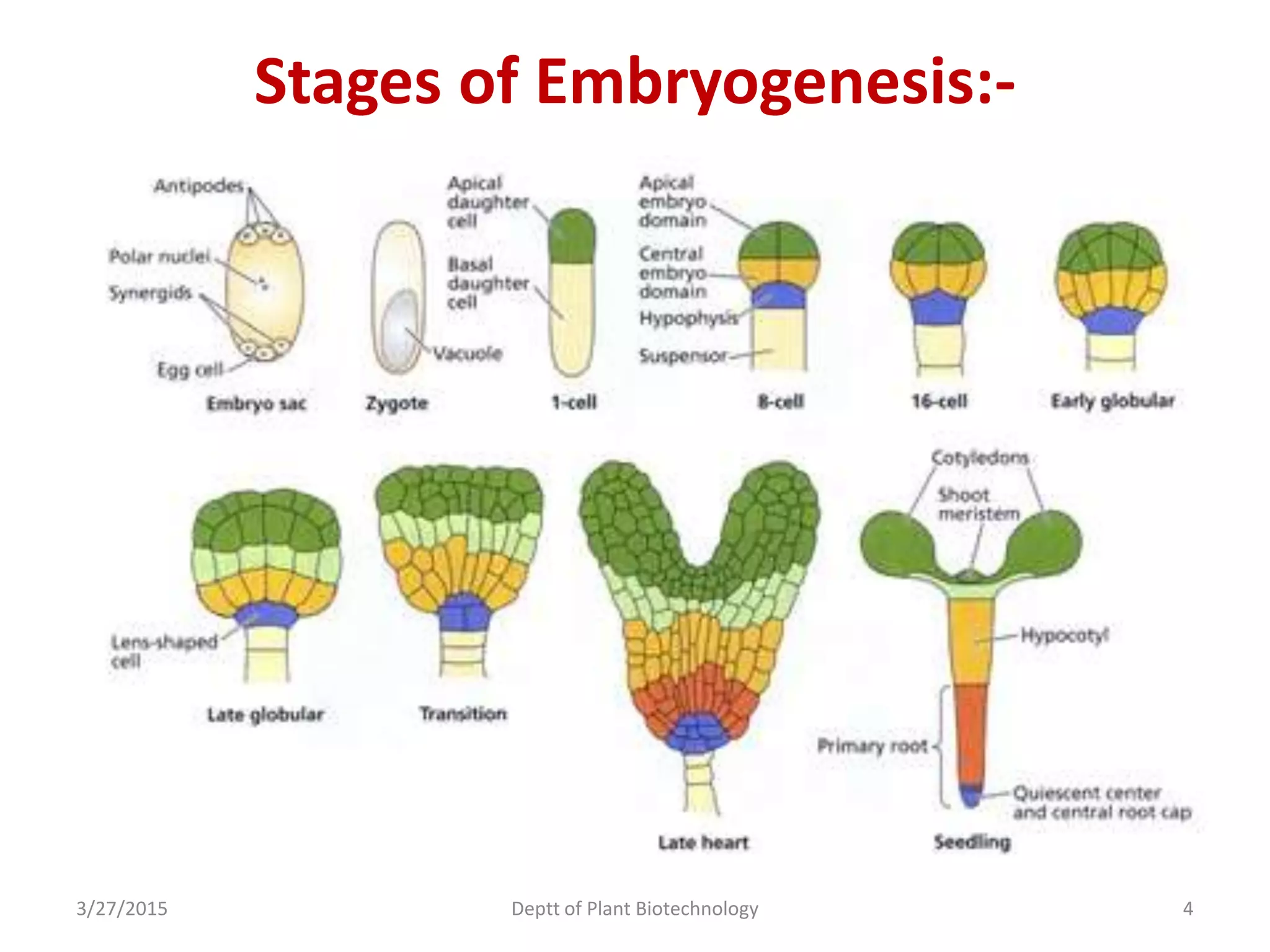
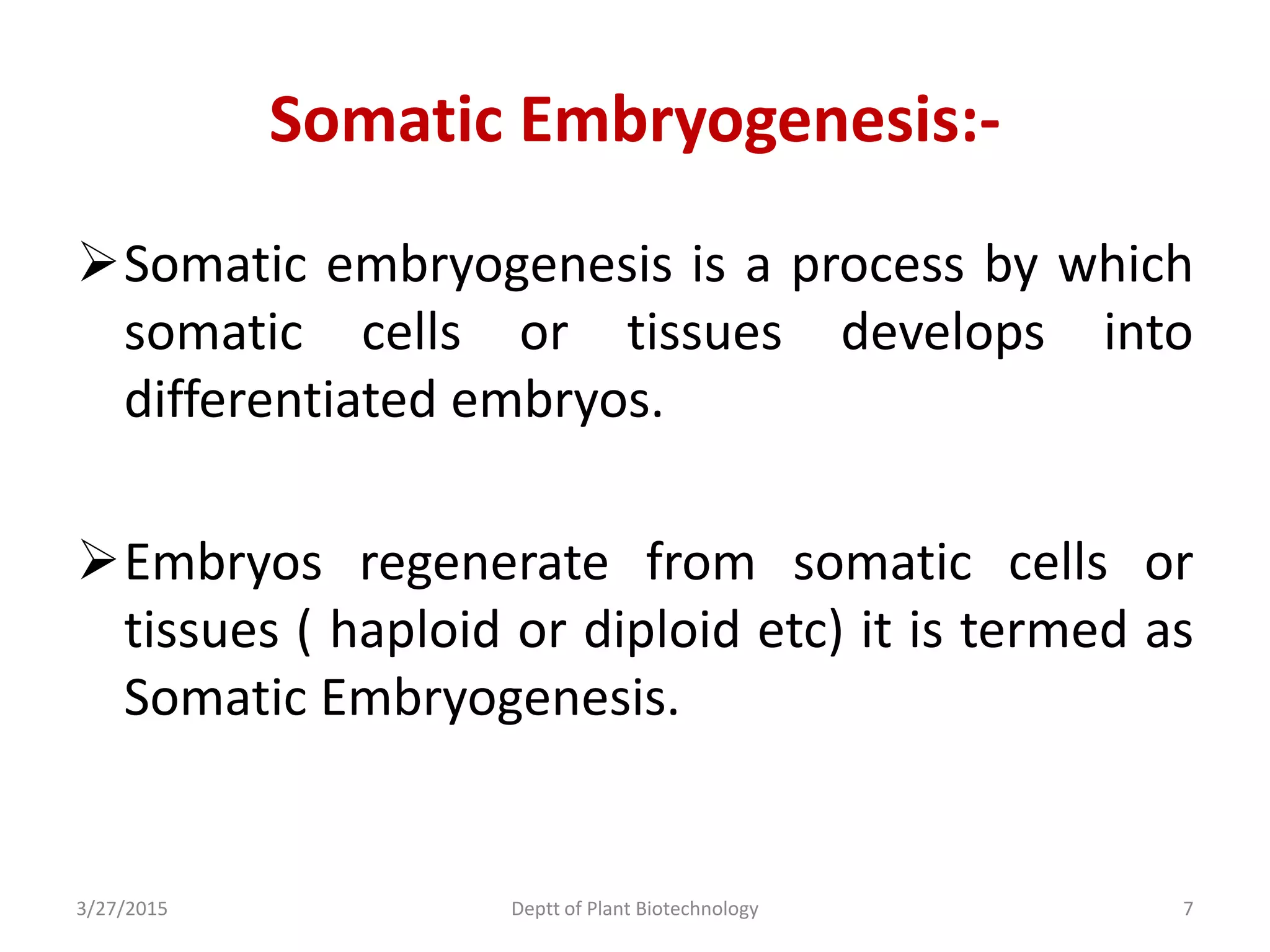
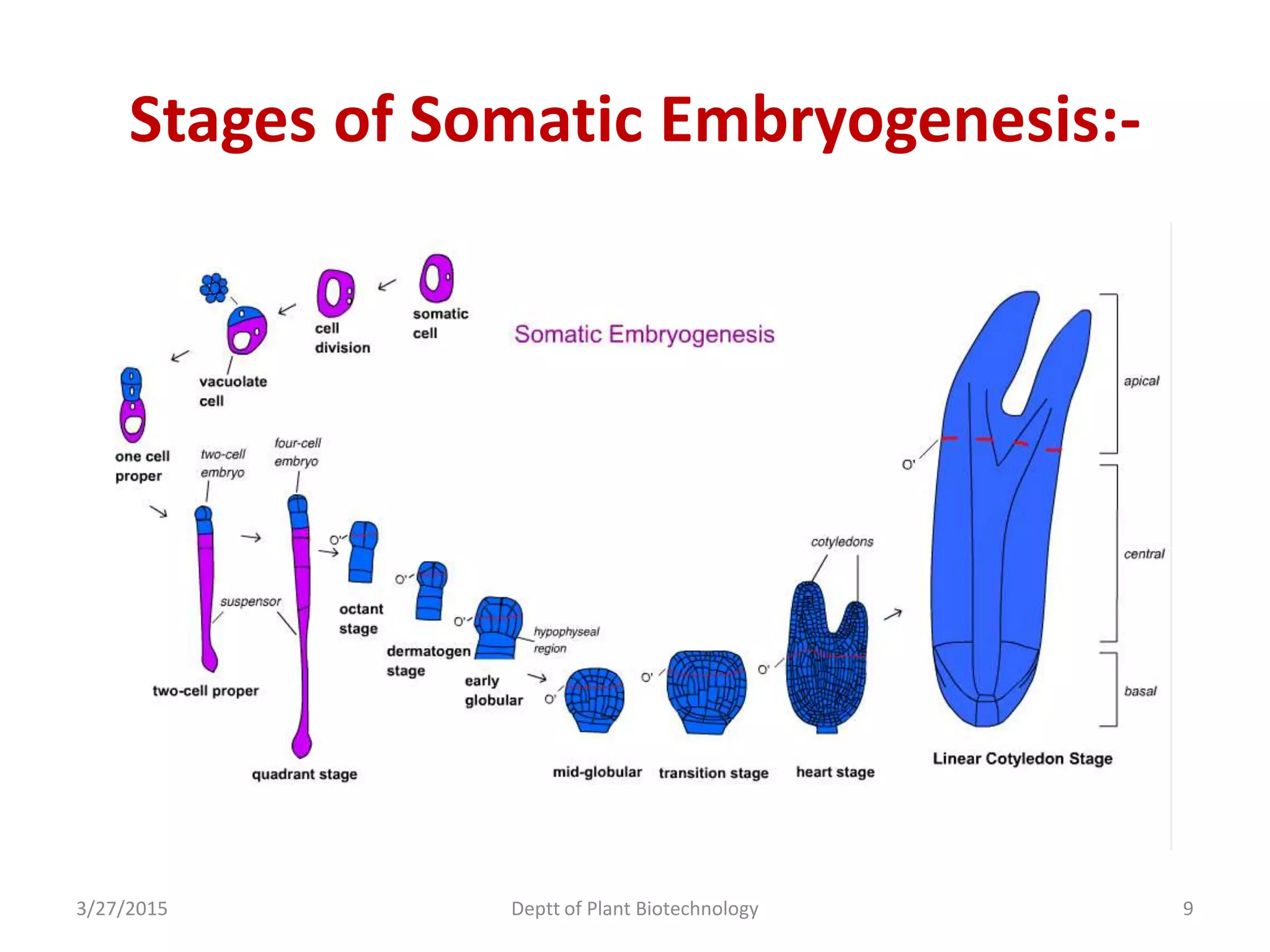
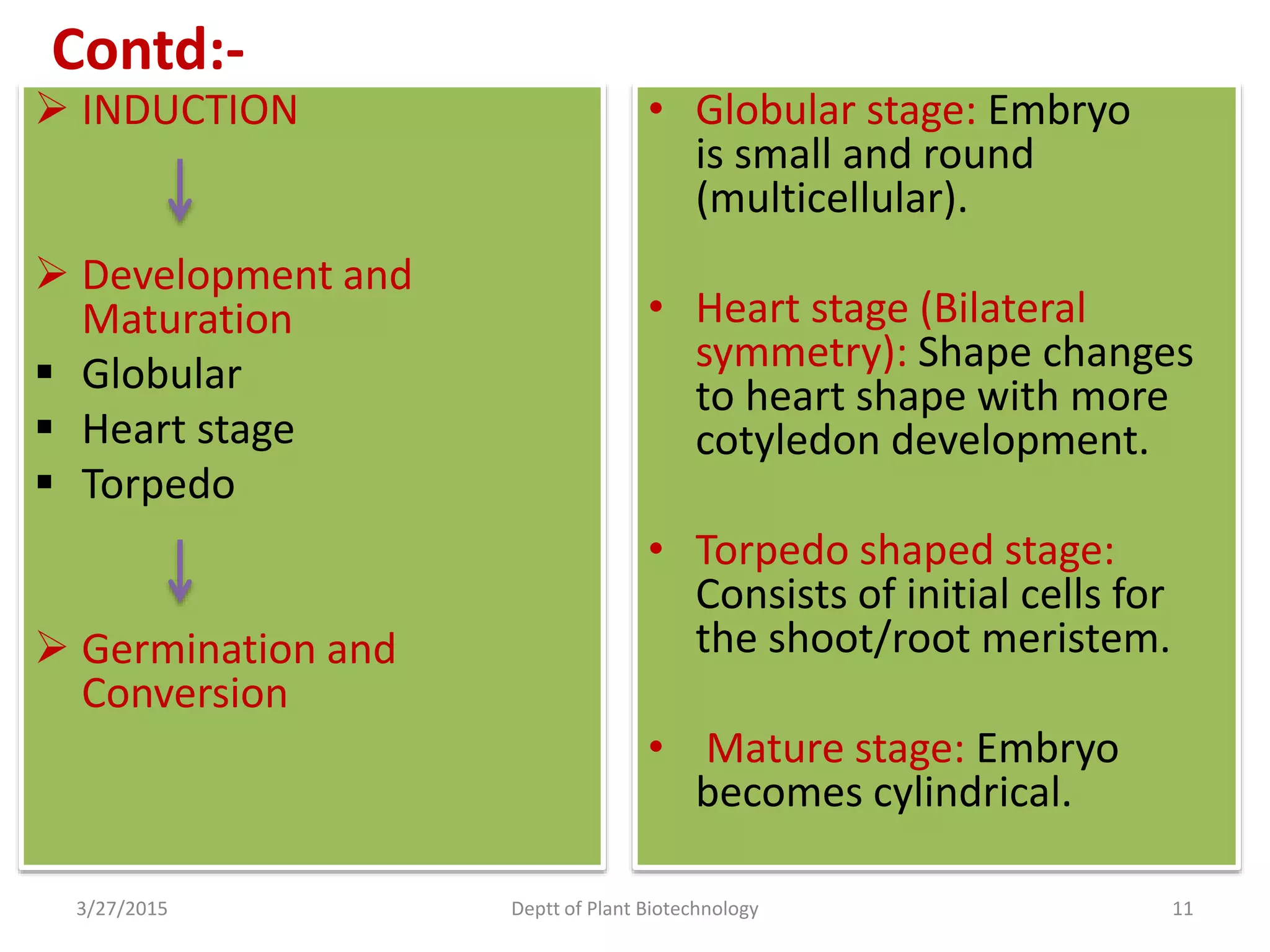
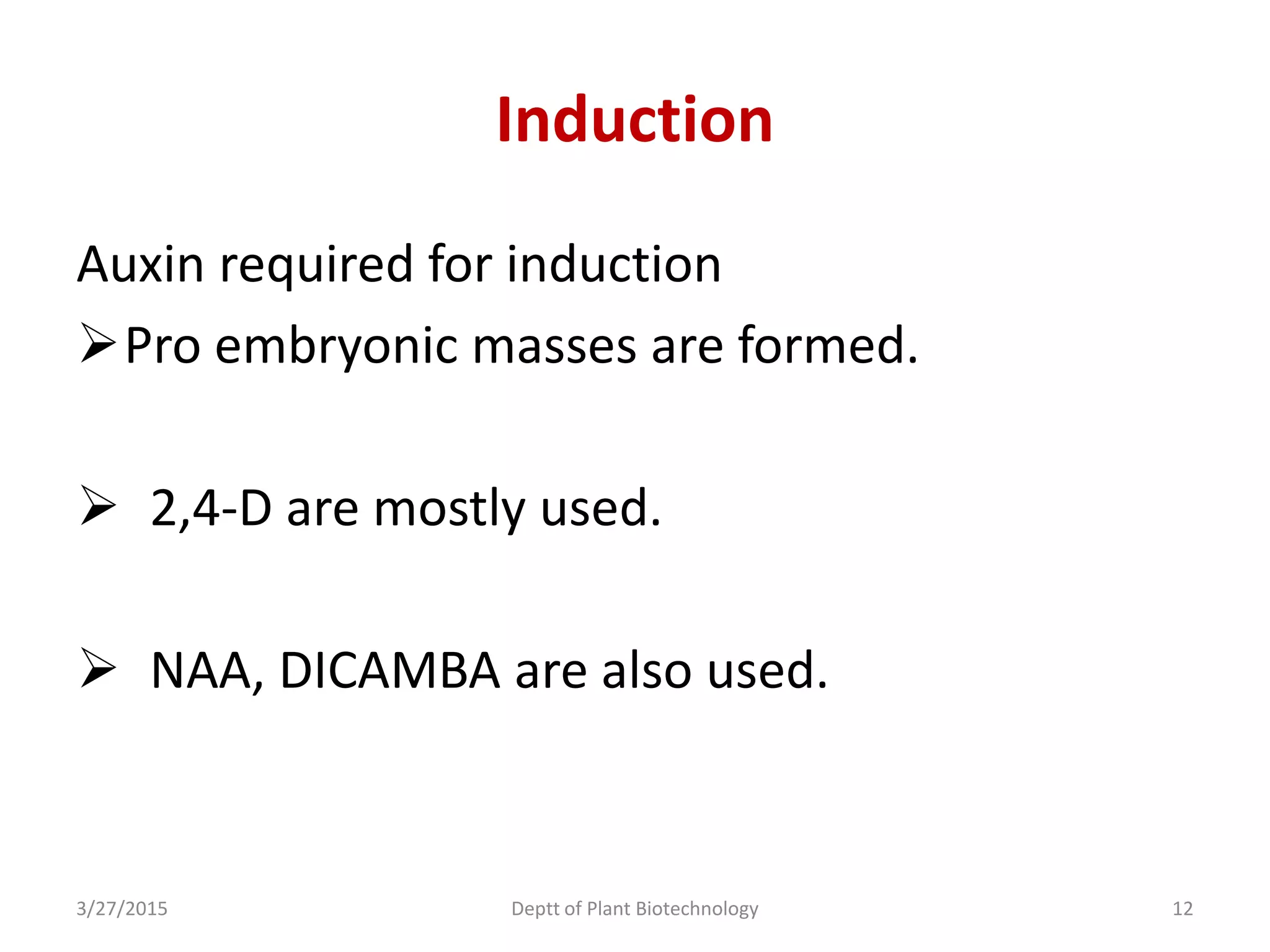
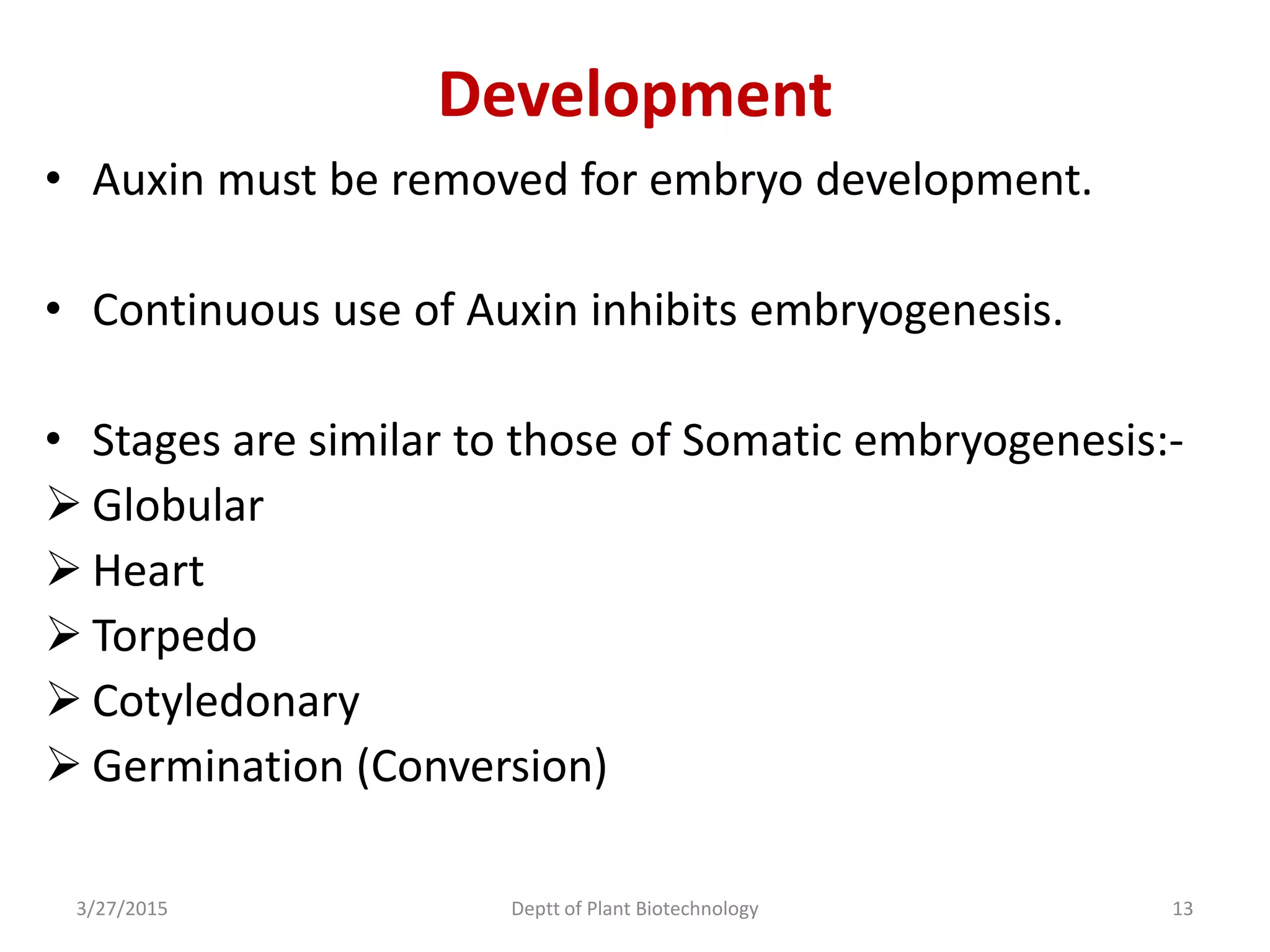
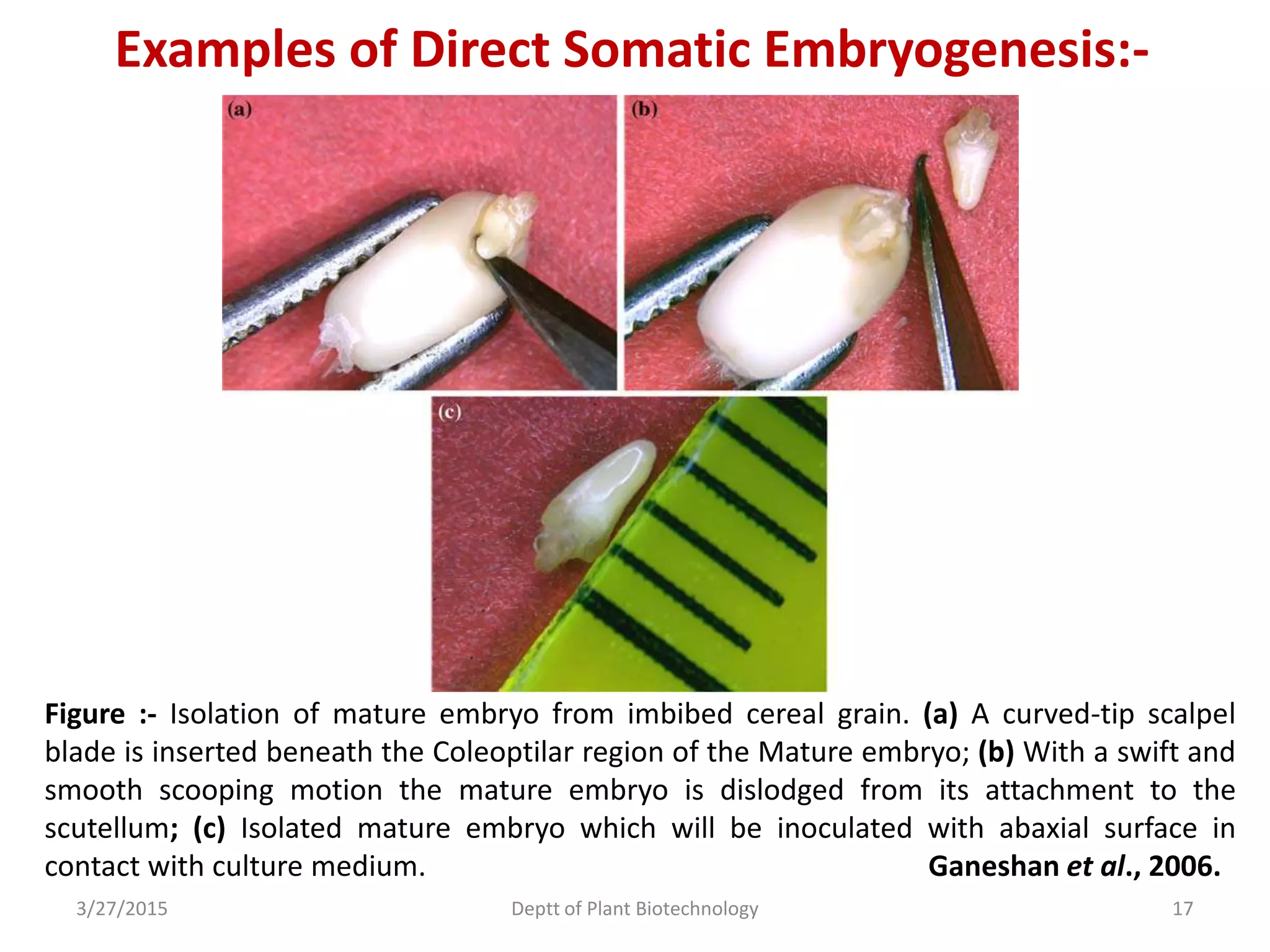
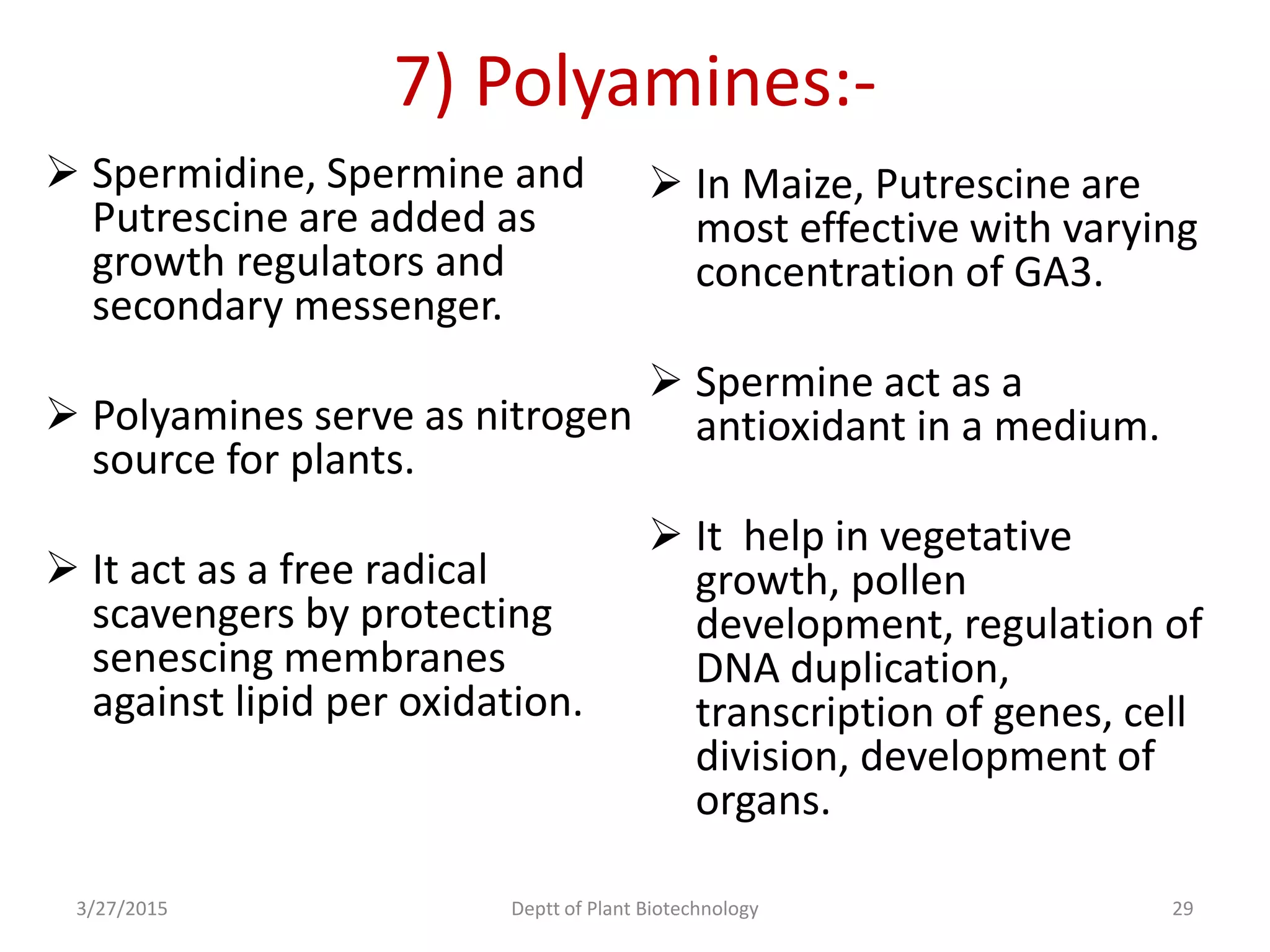
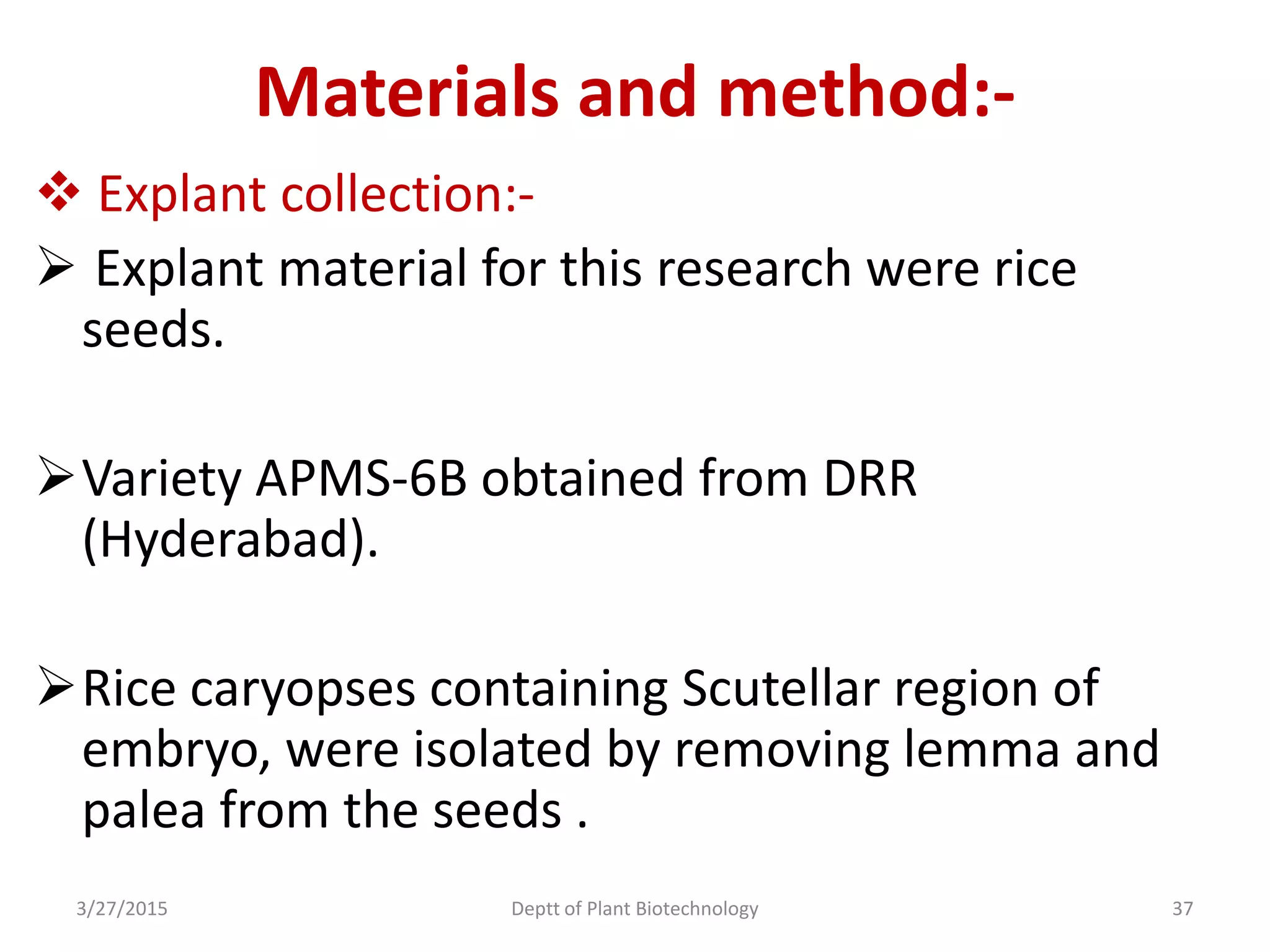
This document summarizes research on somatic embryogenesis in rice. It describes the process of somatic embryogenesis, including the stages of embryogenesis and factors that affect it. The methodology section outlines the materials and methods used, including collecting rice seeds as explants, sterilizing them, and culturing them on callus induction and embryo germination media with different concentrations of plant growth regulators like 2,4-D, BAP and NAA. The goal is to develop an efficient system for somatic embryogenesis and plant regeneration in rice.















![8) Phytosulfokine
It modulate the culture media.
It promote somatic
embryogenesis by activating
cell division of embryogenic
cells, in presence auxin.
Phytosulfokine increases the
cell through differentiation
process.
9) Phenolic compounds:-
Phenolic compounds are inhibit
somatic embryogenesis.
4hydroxy benzyl alcohol inhibits
the globular stages.
Vanillyl benzyl ether are inhibit
the suspensor development.
Recently identification of 4
[(phenyl methoxy) methyl]
phenol involves in seed
development stills unknown.
3/27/2015 Deptt of Plant Biotechnology 30](https://image.slidesharecdn.com/embryogenesis27mar15-150327095746-conversion-gate01/75/Embryogenesis-27-mar-15-30-2048.jpg)





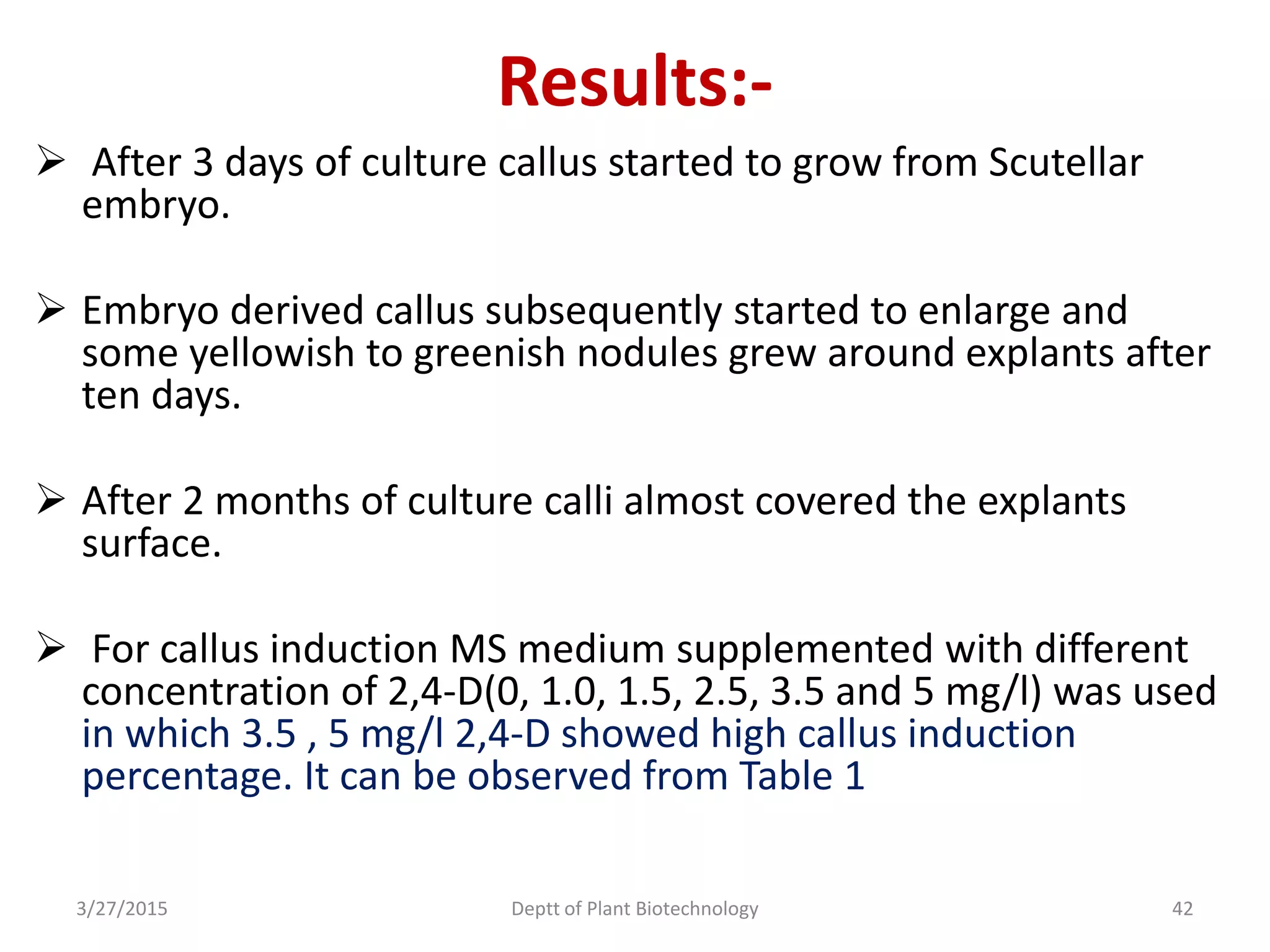
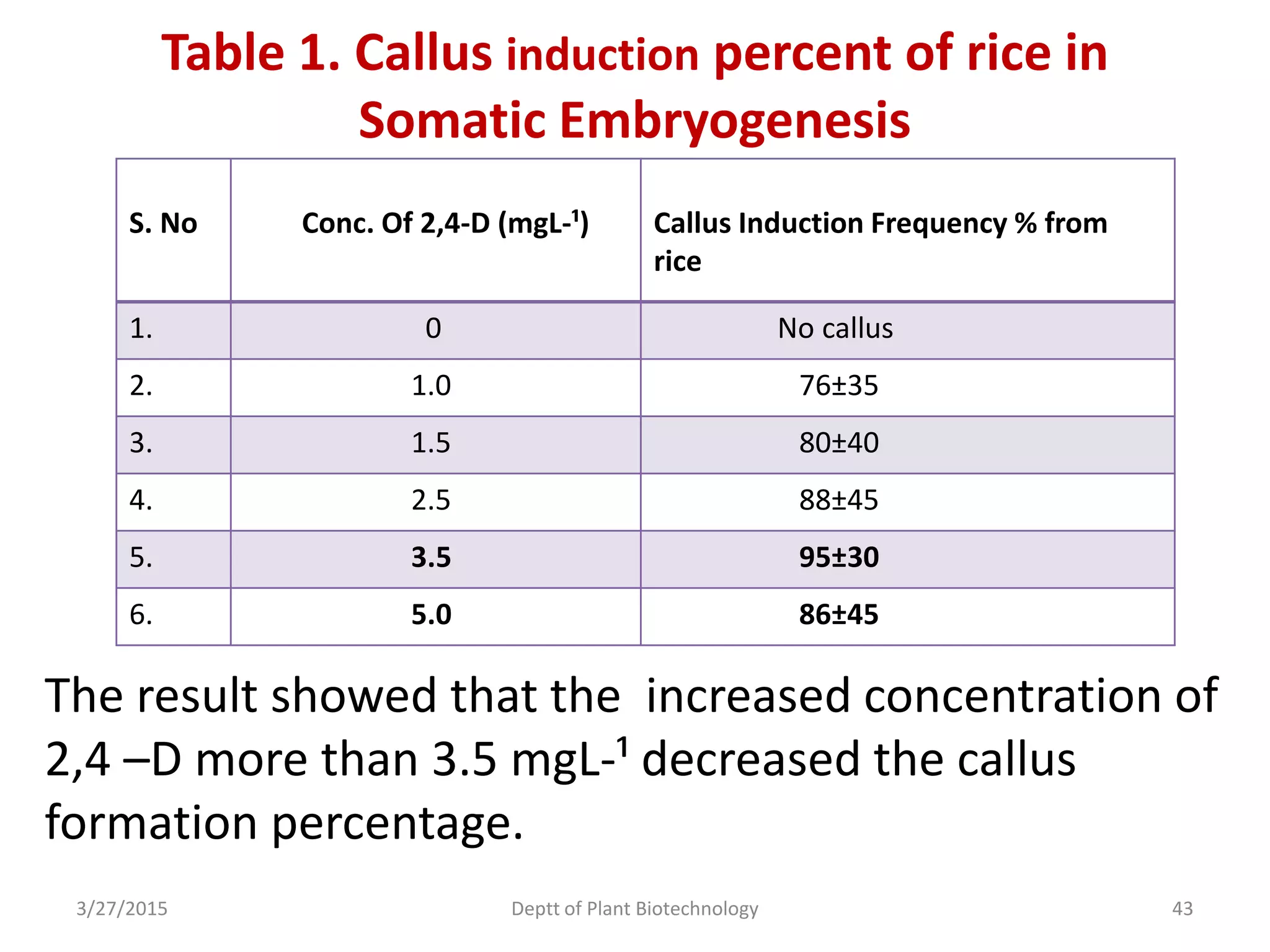
![Callus Induction Media:-
Different concentrations of 2, 4-D [0.1, 1.5, 2.5,3.5
and 5 mgL-1 (w/v)] were used as the treatments for
embryogenic callus induction.
Media were kept in dark condition for 1 week,
25±2°C at room temperature.
After 1 week transferred the cultures under 16 hrs
lighting , provided by fluorescent bulbs with 15.75
µmolm-²s-¹ for eight weeks.
3/27/2015 Deptt of Plant Biotechnology 40](https://image.slidesharecdn.com/embryogenesis27mar15-150327095746-conversion-gate01/75/Embryogenesis-27-mar-15-40-2048.jpg)