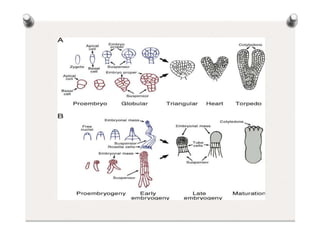
1. Zygotic embryogenesis is the process by which a zygote undergoes differentiation into a mature embryo through cell division and growth.
2. Types of embryos include zygotic embryos formed by fertilization and non-zygotic embryos like somatic and parthenogenic embryos.
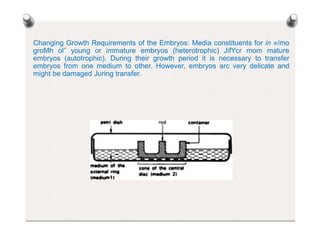
3. In vitro embryogenesis techniques like embryo culture can rescue hybrid embryos from wide crosses that experience post-fertilization abortion, allowing the production of rare hybrids. Embryo culture involves excising embryos and providing a nutrient medium for growth.