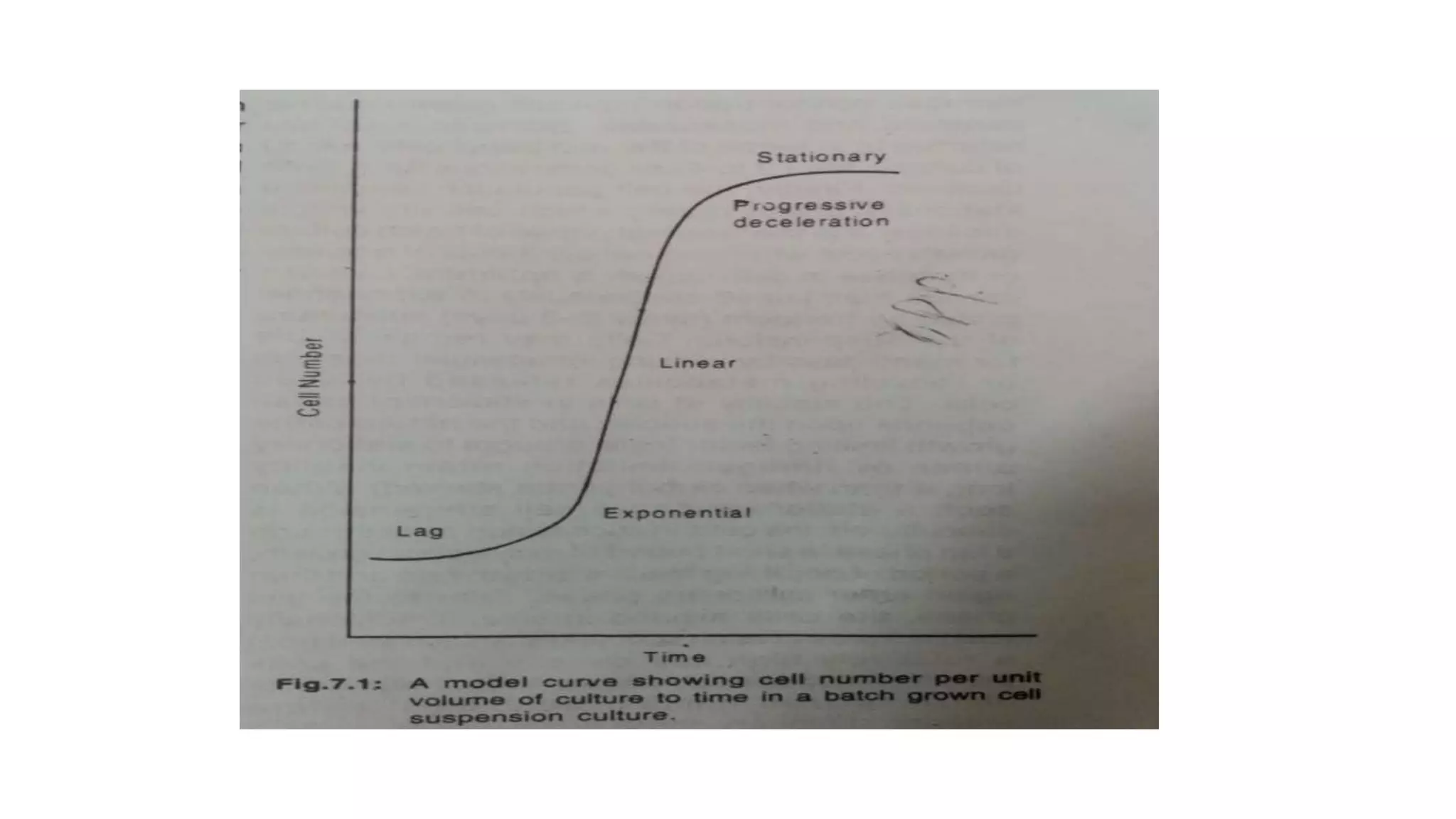
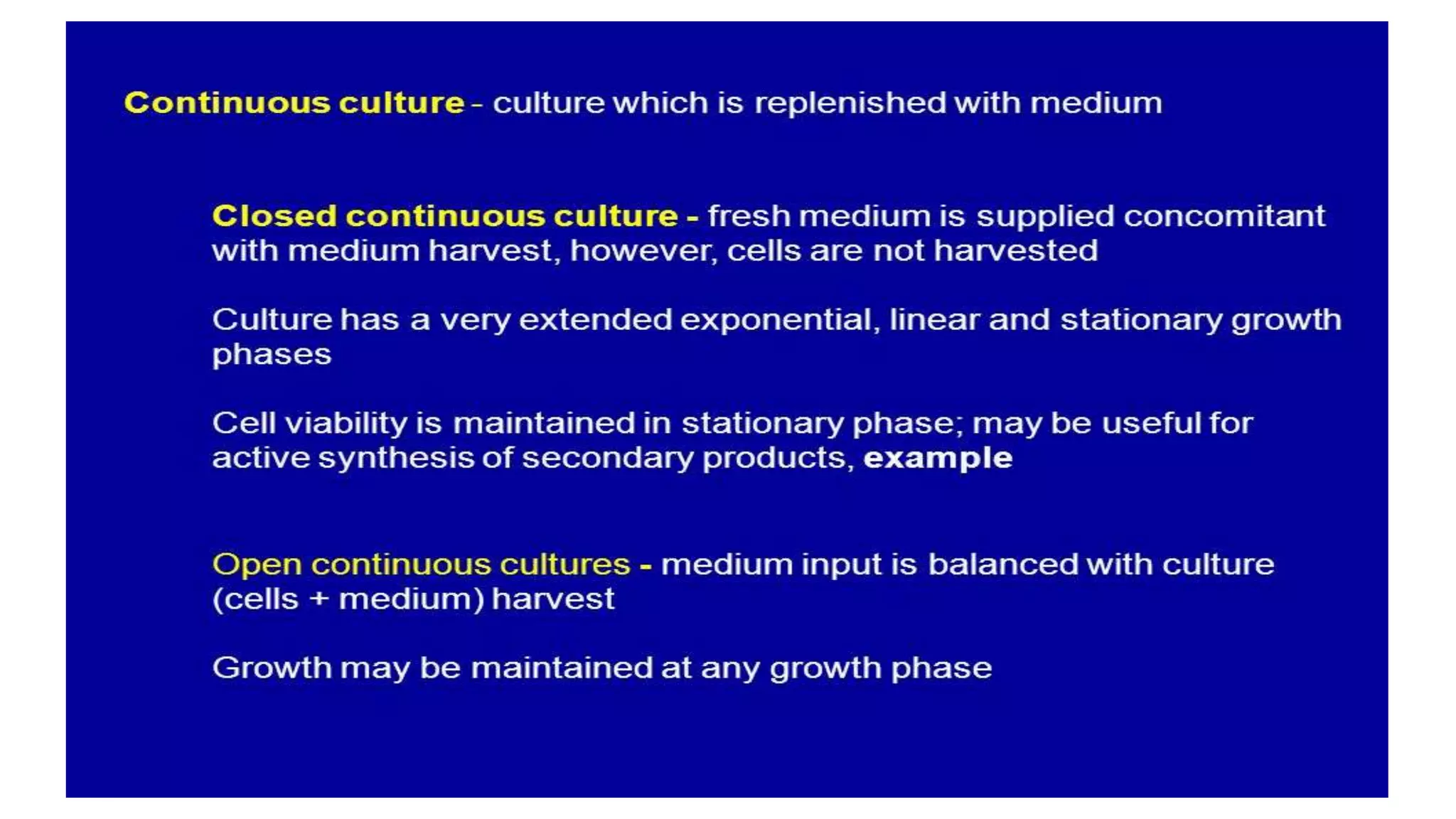
Cell suspension culture involves growing single plant cells or small cell aggregates in agitated liquid medium. It allows studying cellular events during growth and development without the limitations of callus culture. An ideal suspension culture consists of only uniformly growing single cells. It is established by transferring friable callus pieces to agitated medium, then filtering and subculturing the dispersed cells. Suspension culture offers insights into cell physiology and is useful for cloning, secondary metabolite production, and mutagenesis studies. While it addresses issues with callus culture, cell suspension cultures can have decreasing productivity over time and slow growth.