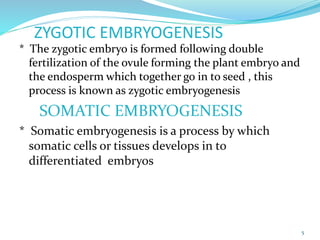
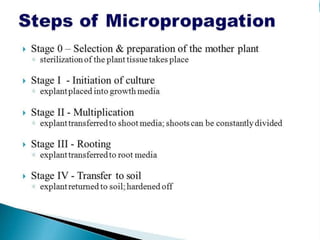
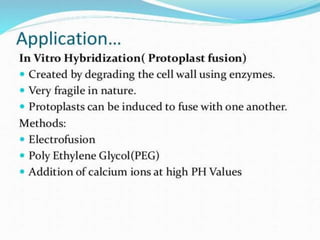
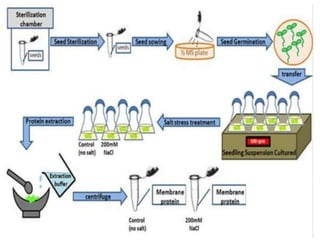
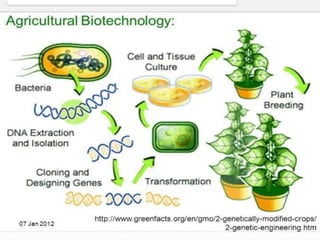
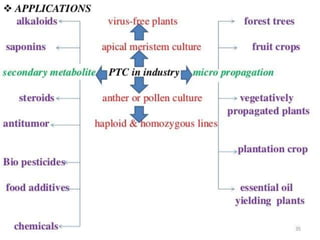
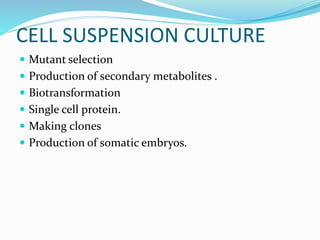
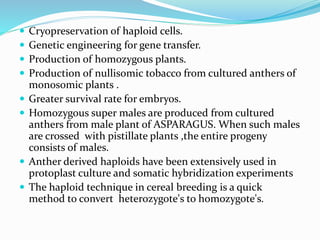
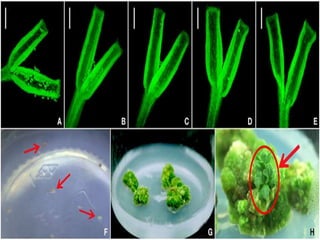
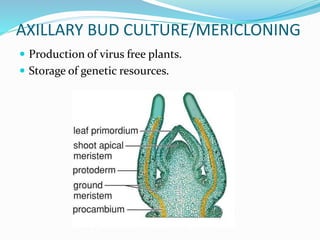
This document summarizes various plant tissue culture techniques including embryogenesis, organogenesis, micropropagation, callus culture, cell suspension culture, anther culture, shoot tip culture, protoplast culture, axillary bud culture, and hairy root culture. It describes the applications of each technique such as clonal propagation, production of haploids, secondary metabolite production, virus elimination, and genetic conservation. Direct and indirect organogenesis are also summarized.