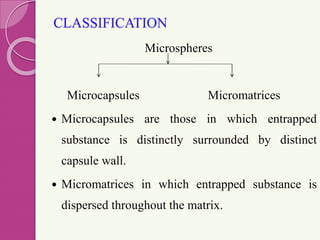
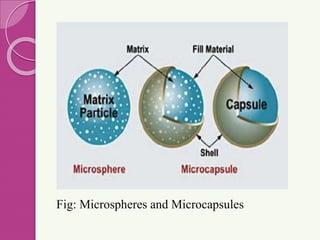
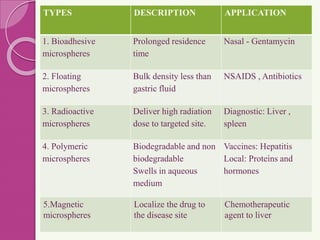
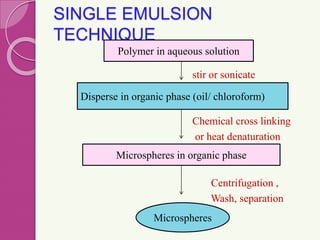
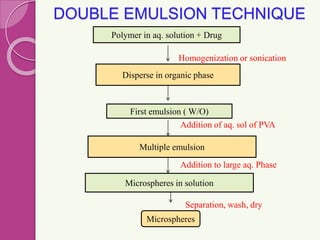
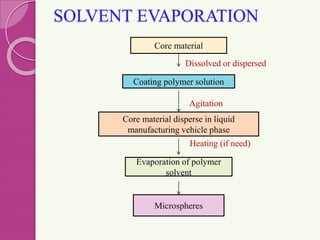
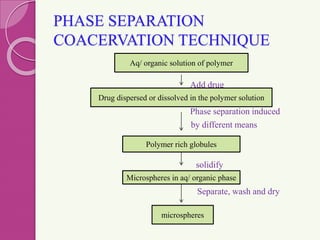
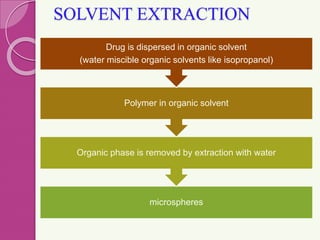
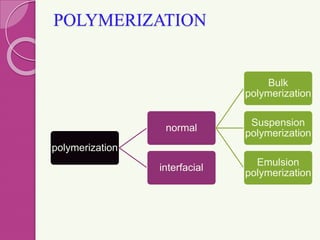
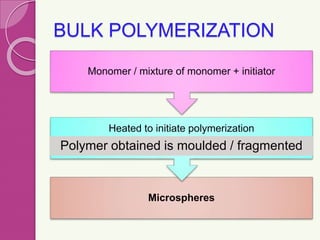
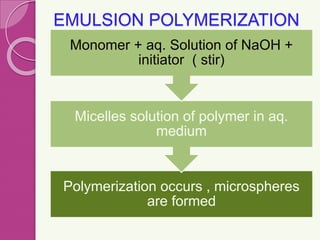
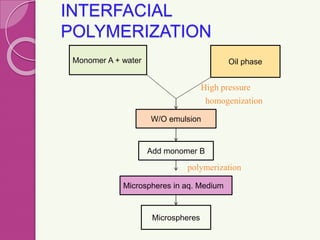
Microspheres are small, biodegradable spherical particles, often made of proteins or synthetic polymers, used for drug delivery to improve bioavailability and therapeutic effects. They come in various types, including bioadhesive and floating microspheres, with various preparation methods and applications such as ophthalmic and transdermal drug delivery. Despite their advantages, microspheres have drawbacks like high costs and stability issues, necessitating thorough evaluation of properties such as size, drug entrapment efficiency, and adhesion.