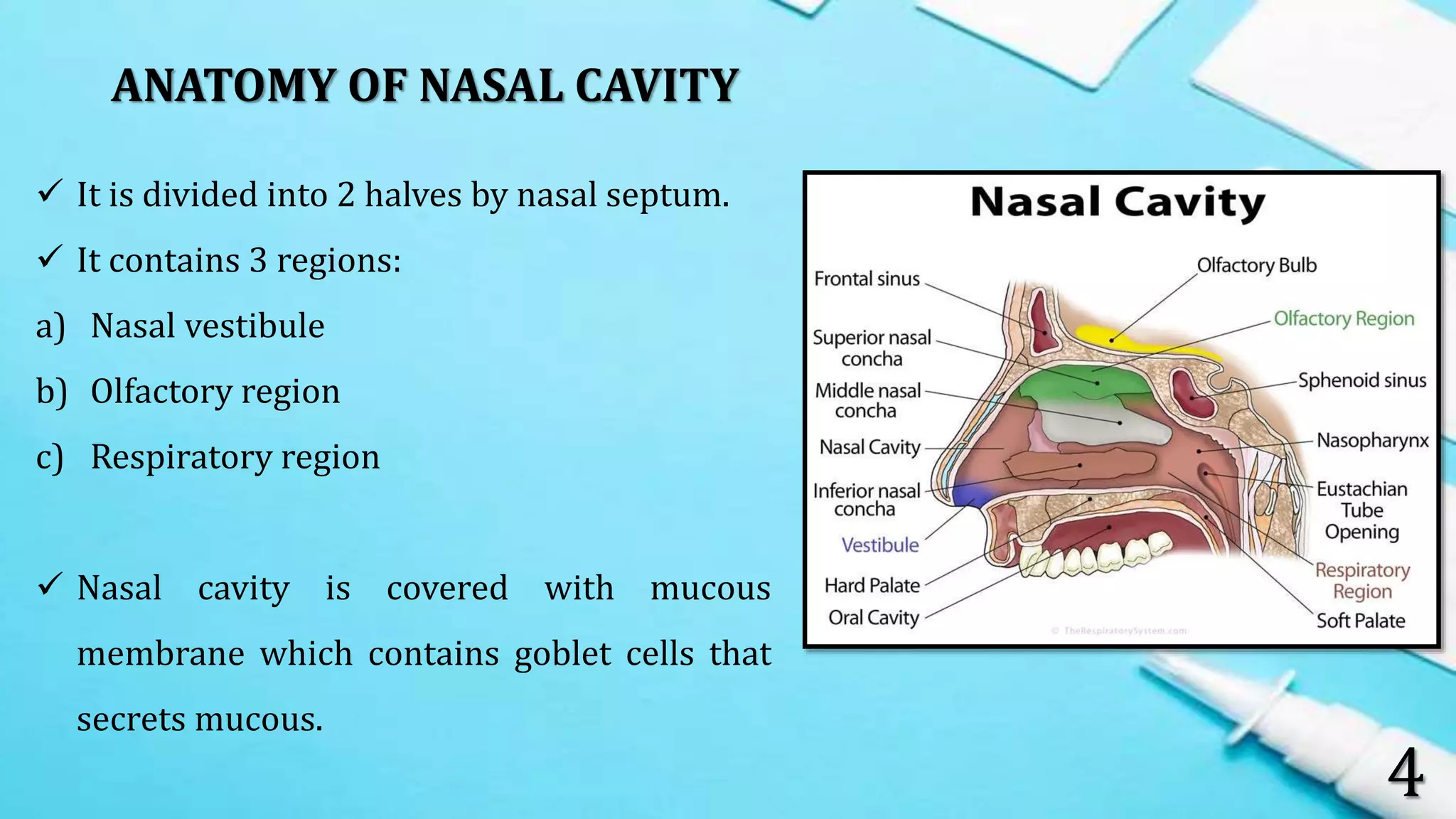
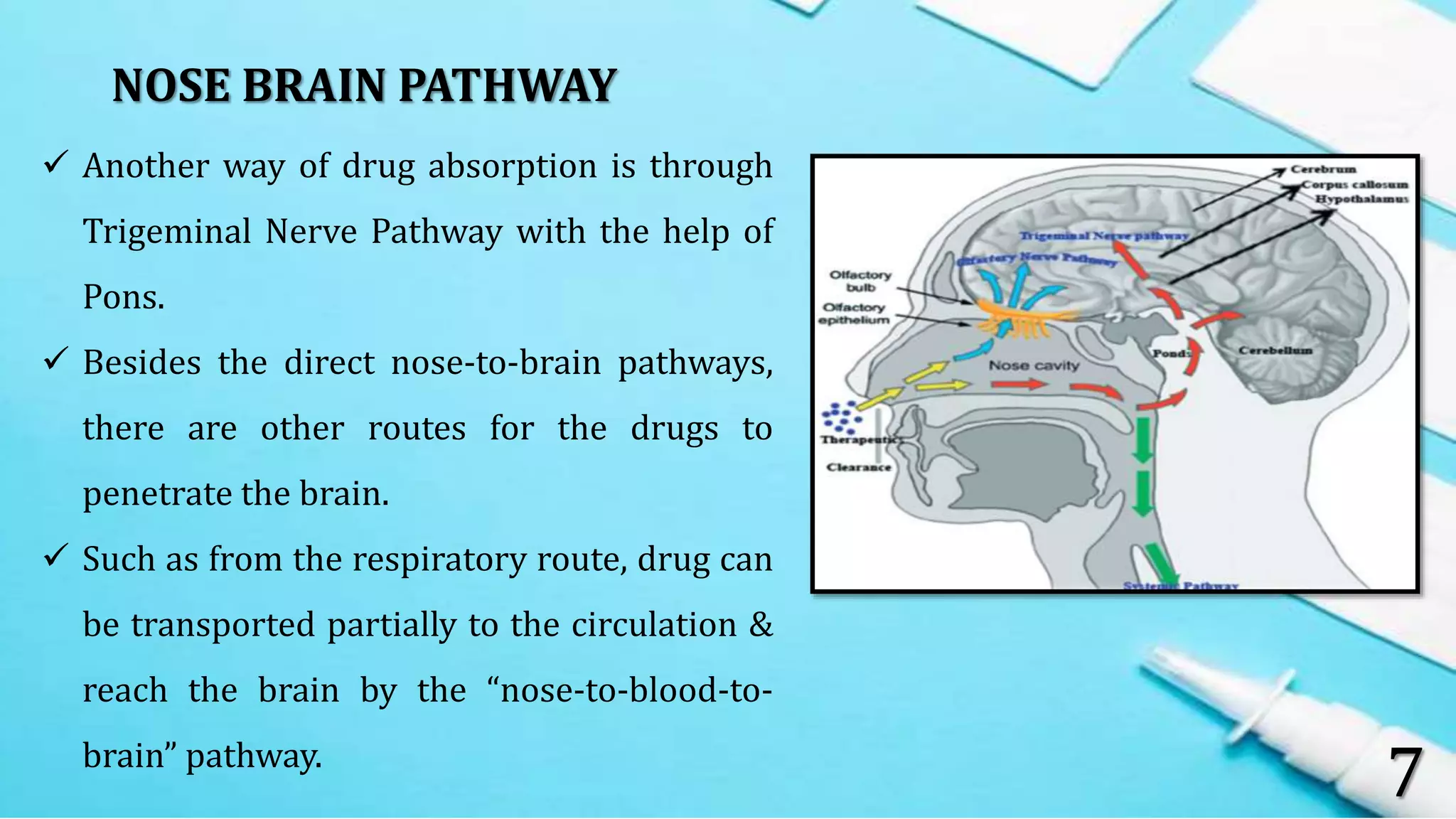
This document provides an overview of intra nasal drug delivery systems. It discusses the anatomy of the nasal cavity, mechanisms of drug absorption such as paracellular and transcellular transport, and factors that affect drug absorption like biological, physiological and formulation related factors. It also describes the advantages and limitations of the nasal route. Various dosage forms for nasal delivery including drops, sprays, gels and powders are mentioned. Evaluation methods like in-vitro and in-vivo studies are summarized. Finally, applications of the nasal route for delivery of peptides, vaccines and CNS drugs are highlighted.