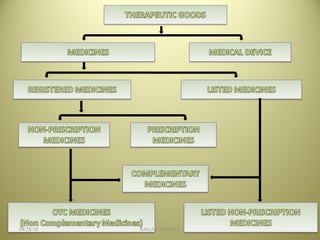
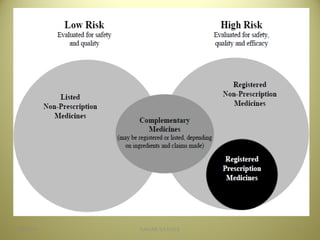
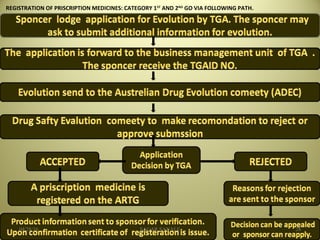
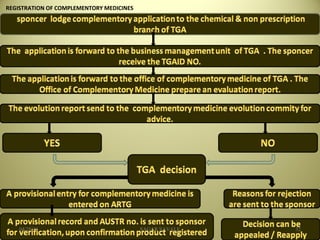
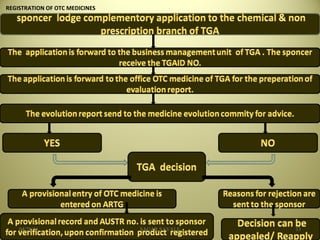
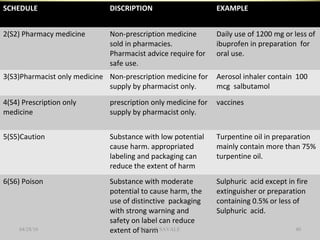
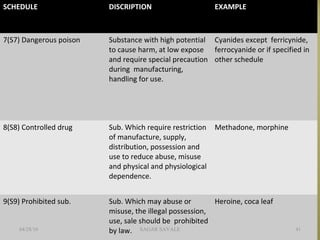
The document outlines the history and regulatory framework of the Therapeutic Goods Administration (TGA) in Australia, which ensures the safety, quality, and efficacy of therapeutic goods. It describes the structure of the TGA, the processes for evaluating, approving, and monitoring medicines, and distinguishes between registered and listed medicines based on risk levels and regulatory requirements. Additionally, it details the implications of the Therapeutic Goods Act 1989 and the classification of products as medicines or foods based on their claims and intended use.
![Therapeutic Good Administration
Australia [TGA]
1
Mr. Sagar Kishor SavaleMr. Sagar Kishor Savale
Department of Pharmaceutics
avengersagar16@gmail.com
2015-016
Department of Pharmacy (Pharmaceutics)Department of Pharmacy (Pharmaceutics) || Sagar savaleSagar savale
04/28/16 SAGAR SAVALE](https://image.slidesharecdn.com/therapeuticgoodadministration-160428055841/75/Therapeutic-good-administration-1-2048.jpg)