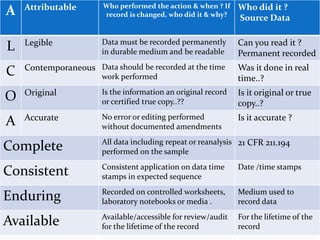
The document outlines the importance of data integrity in compliance with regulatory requirements, defining it as the accuracy, completeness, and consistency of data throughout its lifecycle. It discusses common data integrity issues, including inadequate controls and incomplete records, and highlights recent regulatory changes affecting data management practices. Finally, it emphasizes the need for effective internal audits and training to maintain data integrity and prevent compliance violations.