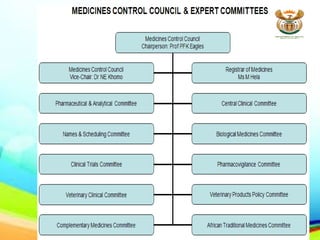
The document provides an overview of various regulatory authorities, including the Therapeutic Goods Administration (TGA) in Australia, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK, and the Medicines Control Council (MCC) in South Africa, detailing their roles in ensuring the safety and efficacy of therapeutic goods. It discusses the regulatory programs, guidelines, and organizational structures involved in the assessment and monitoring of medicines and medical devices, emphasizing the importance of compliance with good manufacturing practices. The conclusion highlights the need for a robust regulatory framework to maintain consumer confidence in the quality and safety of complementary medicines.
![REGULATORY AUTHORITIES
TGA, MHRA, MCC, MCA
Mr. Sagar Kishor savale
[Department of Pharmaceutics]
avengersagar16@gmail.com
Department of Pharmacy (Pharmaceutics) | Sagar savale](https://image.slidesharecdn.com/tgamhramcc-160426055249/85/TGA-MHRA-MCC-MCA-1-320.jpg)