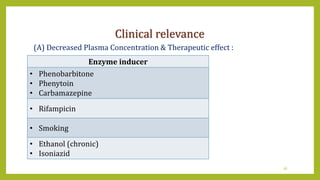
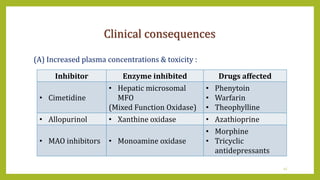
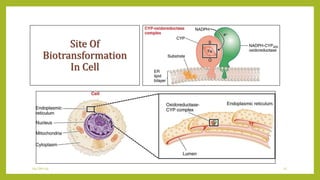
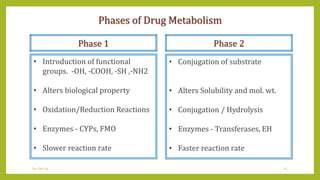
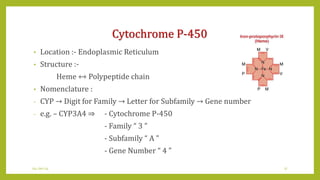
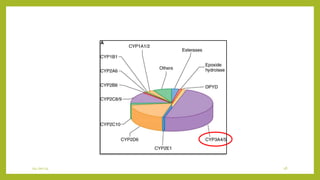
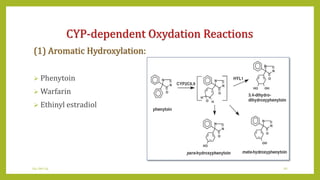
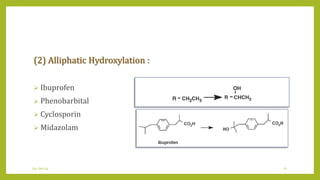
This document discusses biotransformation, or the metabolic processes by which living organisms transform xenobiotics like drugs. It covers the phases of drug metabolism including phase I reactions like oxidation and reduction and phase II reactions like glucuronidation and sulfation. Key enzymes involved in biotransformation are cytochrome P450 enzymes and UDP-glucuronosyltransferases. The document also discusses factors that can influence drug metabolism like age, sex, nutrition, disease states, genetic variations and drug-drug interactions.













![Methylation
• Cofactor : - S-Adenosylmethionine [ SAM ]
• Enzyme : - Methyltransferase ( MT ) (cytosol)
• Highly substrate specific
• Drugs :
- TPMT--- Azathioprine, 6-MT, thioguanine
- COMT --- dopamine, methyl dopa, nor- epinephrine
- HNMT --- Histamine
33](https://image.slidesharecdn.com/metabolism-210805054254/85/Metabolism-33-320.jpg)