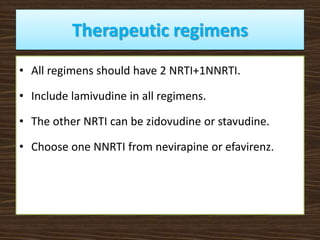
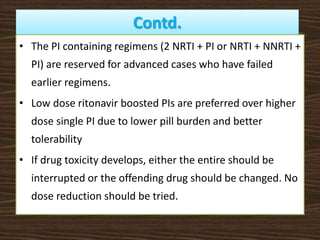
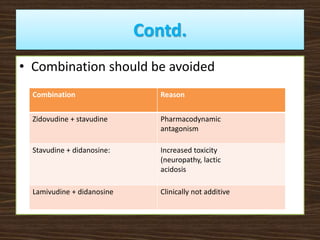
This document provides guidelines for the treatment of HIV infection. It discusses that treatment is complex, requires commitment from the patient, and is lifelong. The goal of highly active antiretroviral therapy (HAART) is to inhibit viral replication, prevent complications and transmission, decrease resistance, and prolong survival. It recommends initiating ART for symptomatic patients, those with CD4 counts below 350, coinfections like HBV/HCV, and pregnant women. First line regimens combine two NRTIs with one NNRTI, including lamivudine. Guidelines are provided for treatment failure, opportunistic infections, pregnancy, and post-exposure prophylaxis. Adherence is key to treatment success.