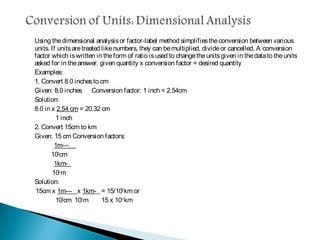
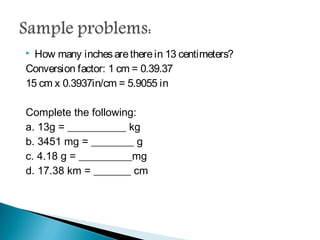
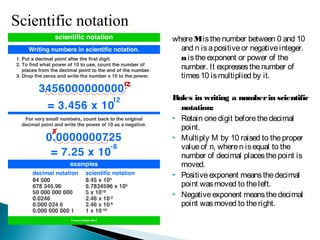
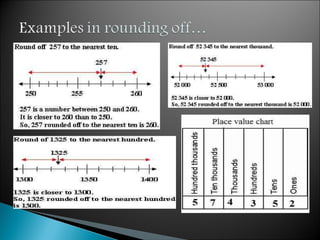
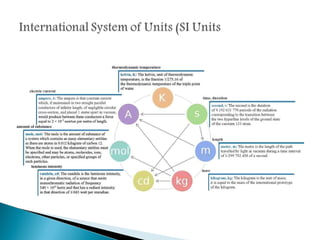
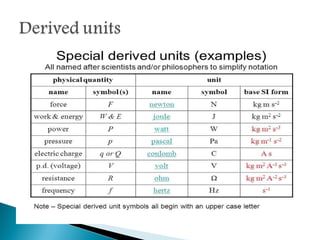
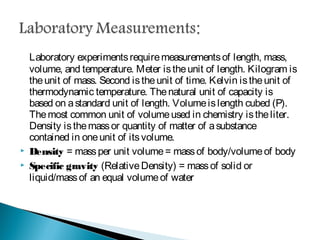
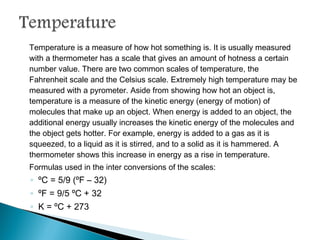
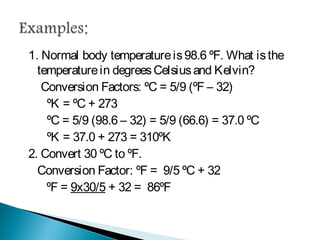
Measurement is the basis of all scientific work, as there are standardized units to quantify different properties like length, mass, time, and others. Various methods like dimensional analysis and factor-label simplify conversions between units by treating them as numbers that can be multiplied or divided. Understanding significant figures and rounding properly is also important when making measurements and calculations in science.