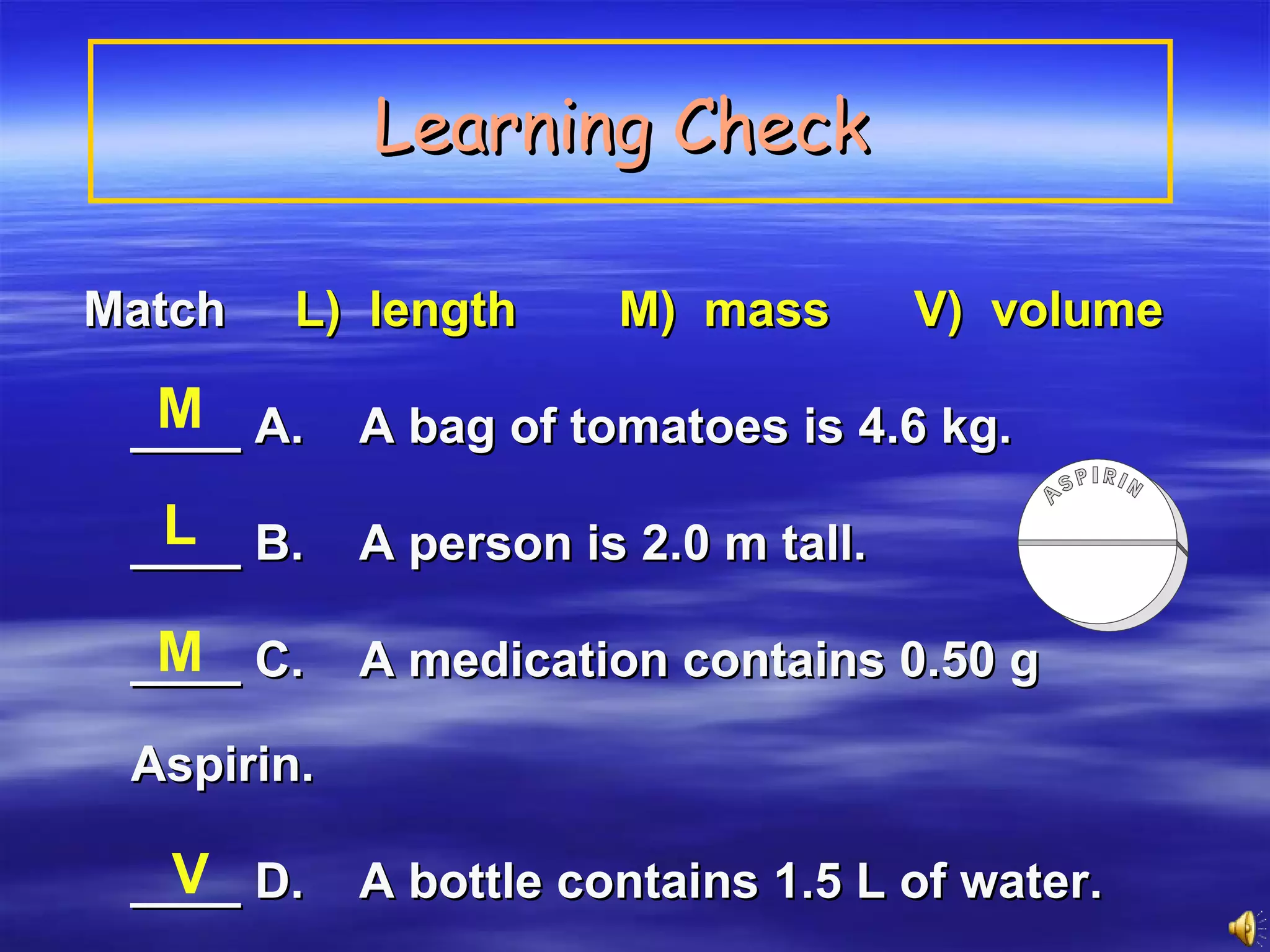
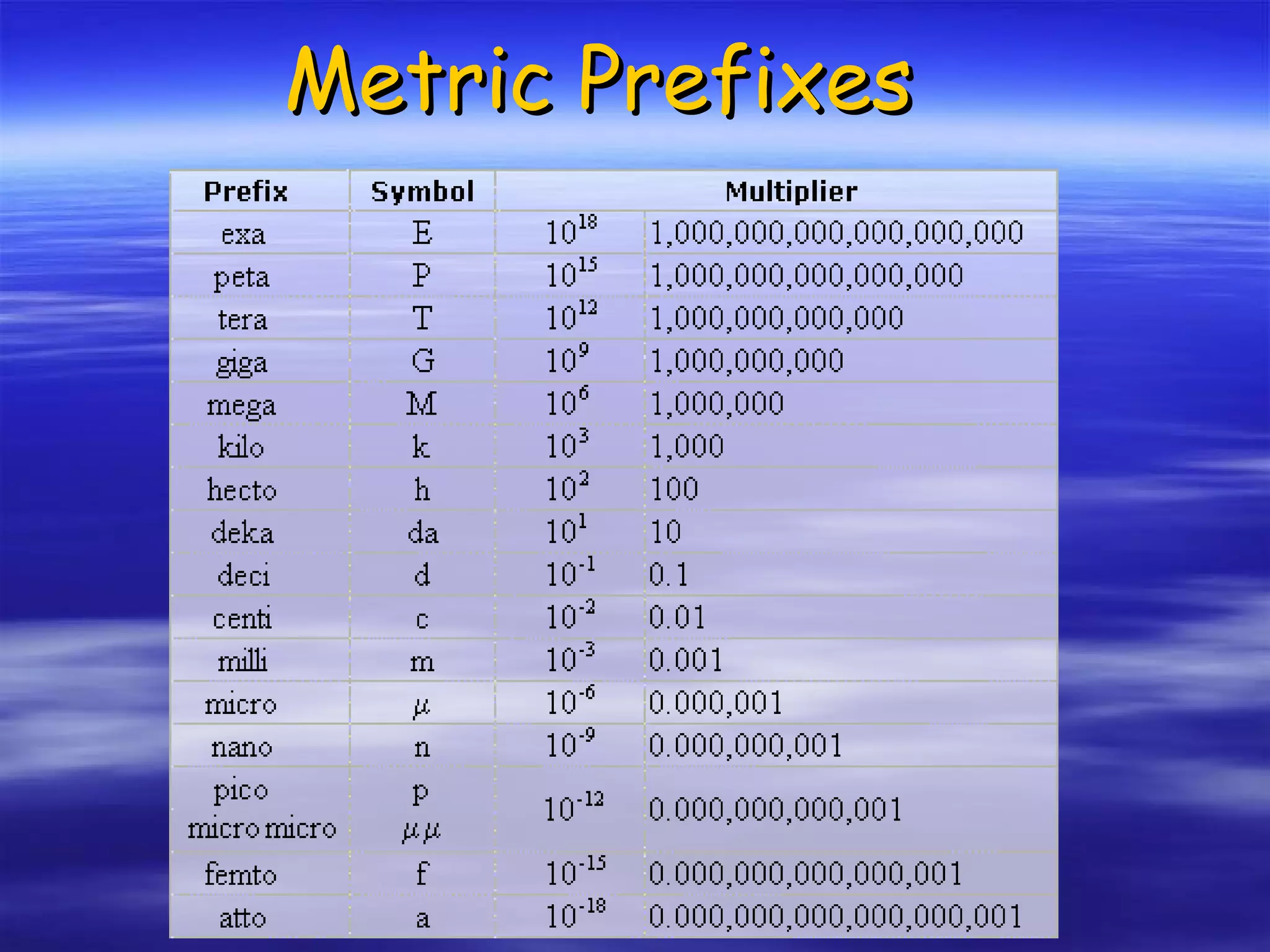
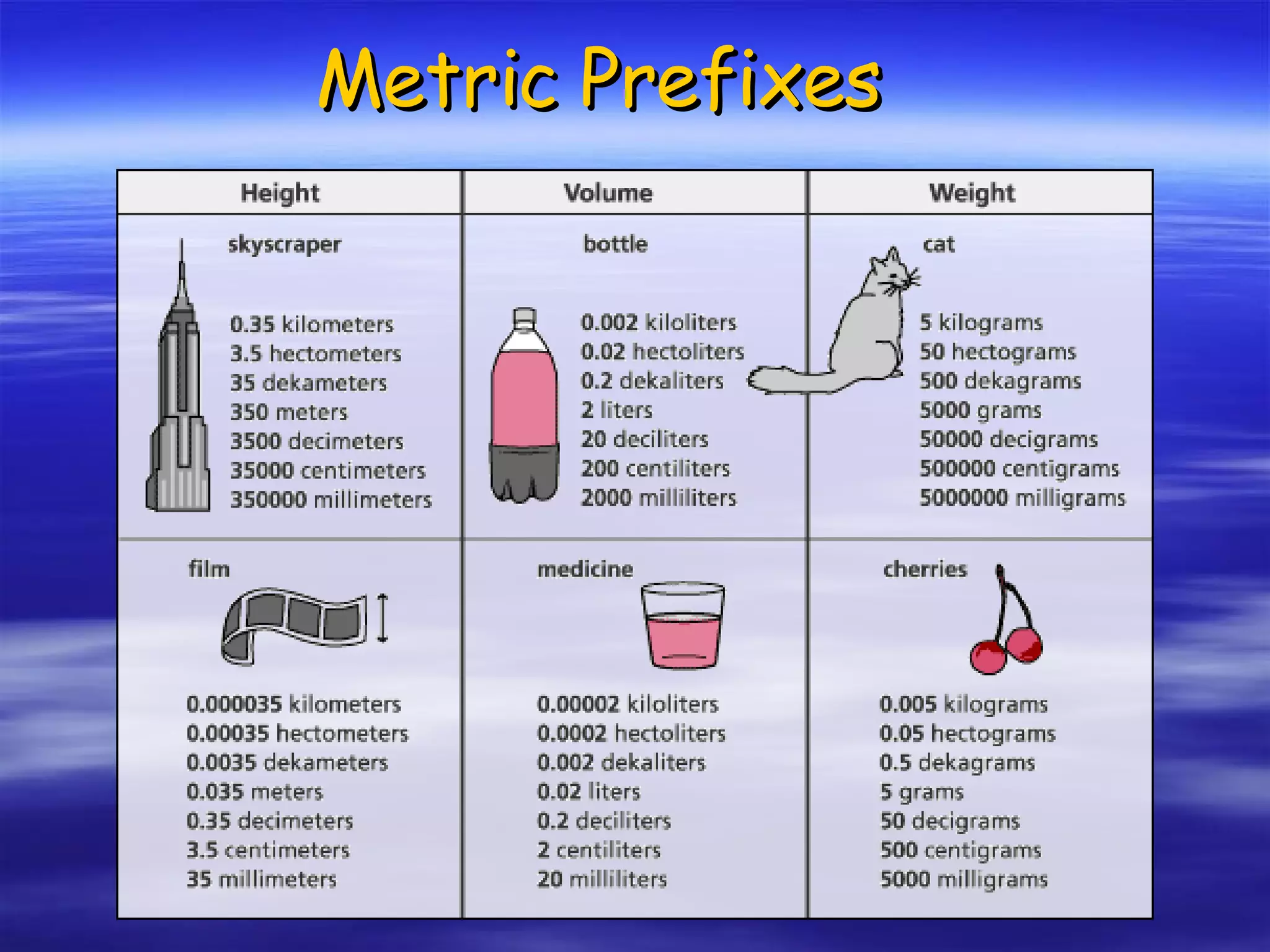
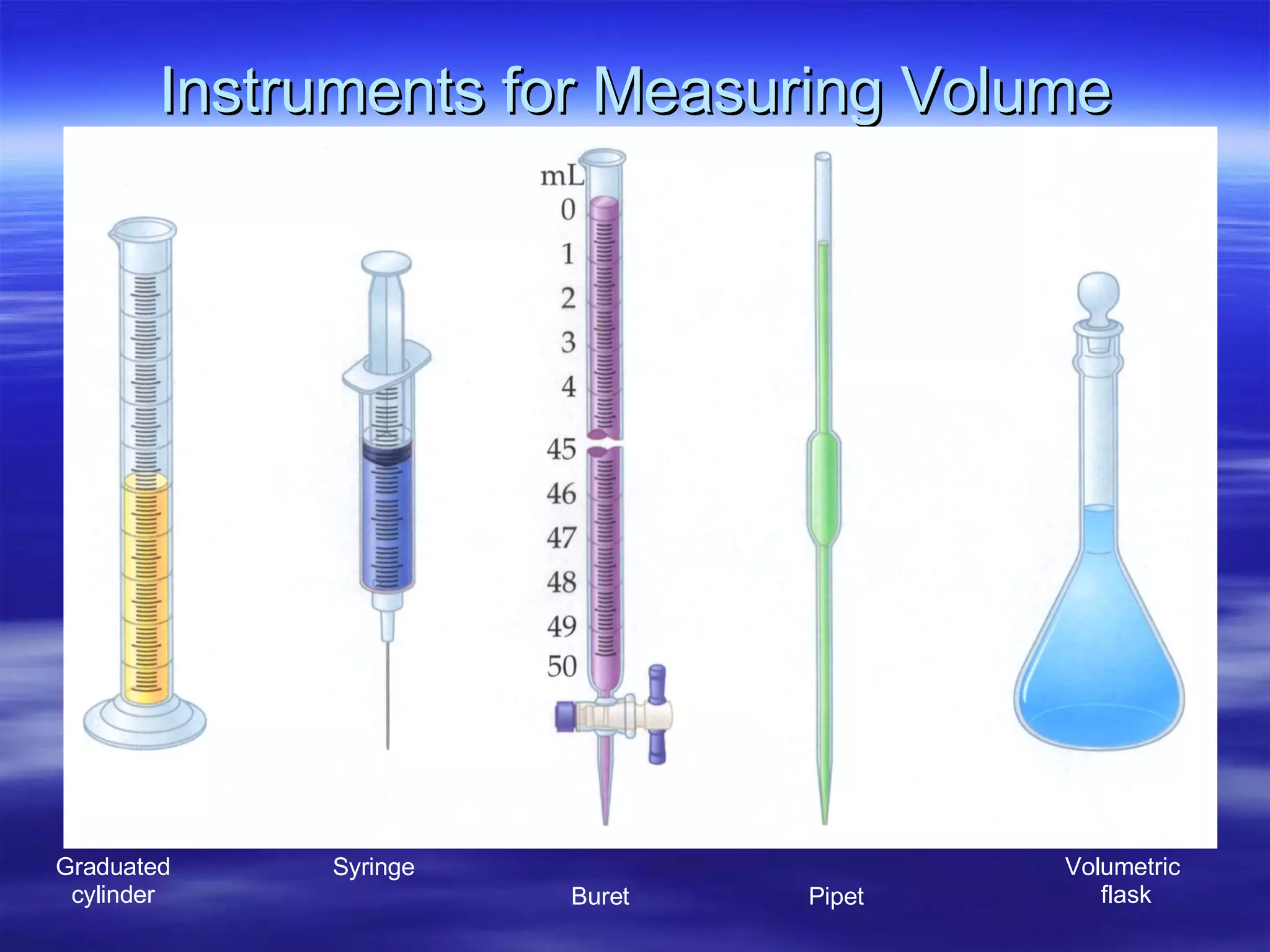
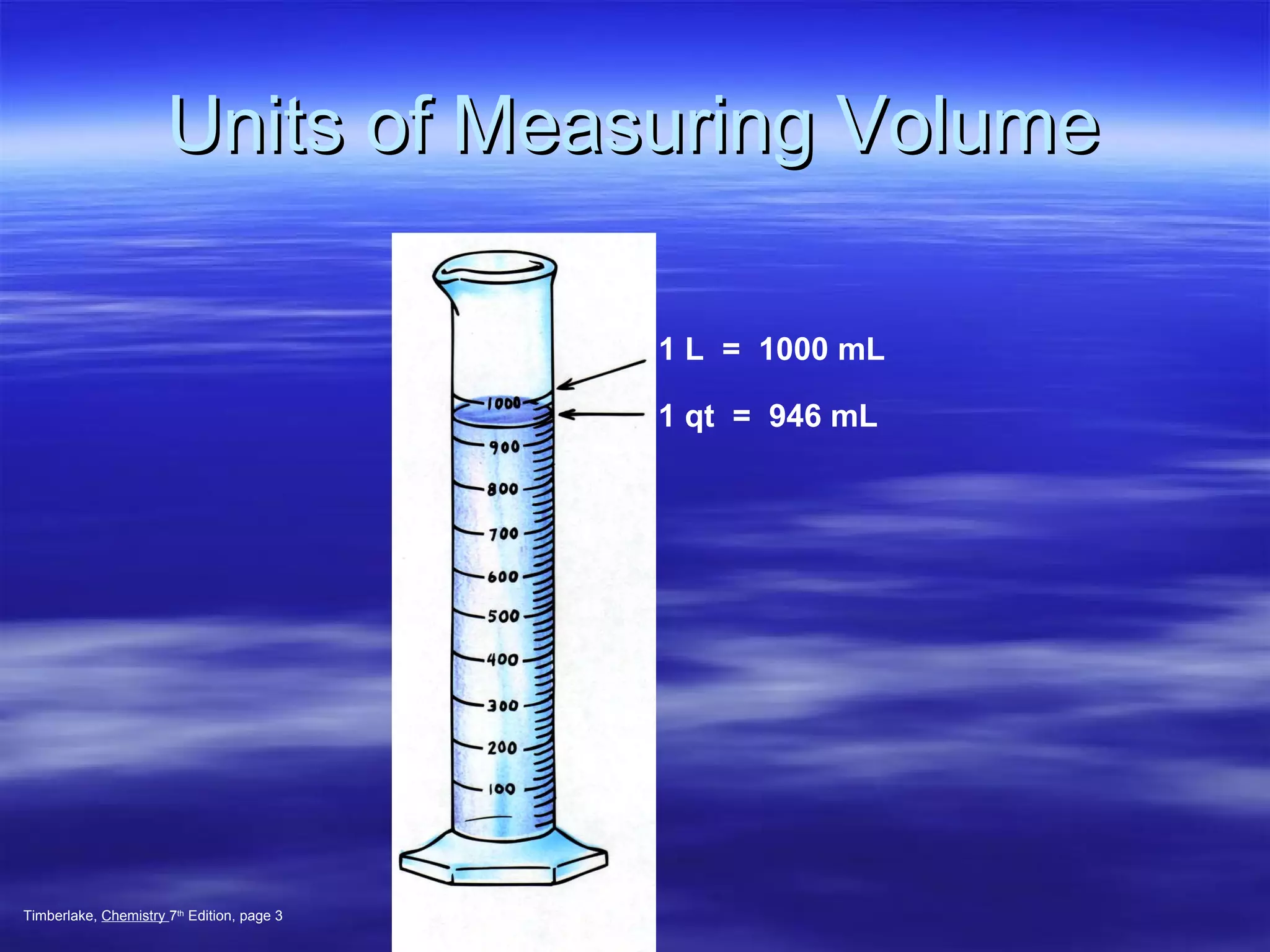
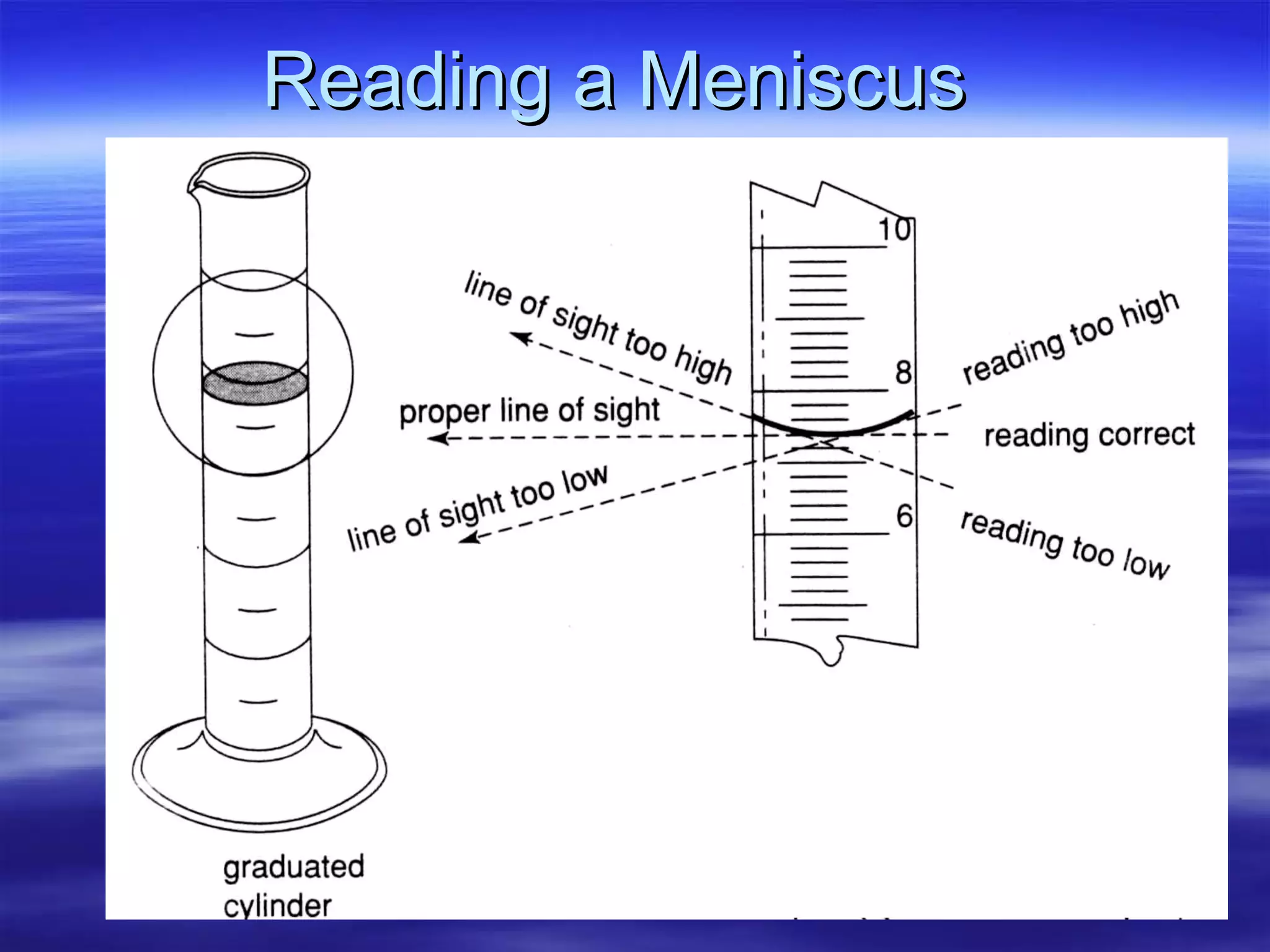
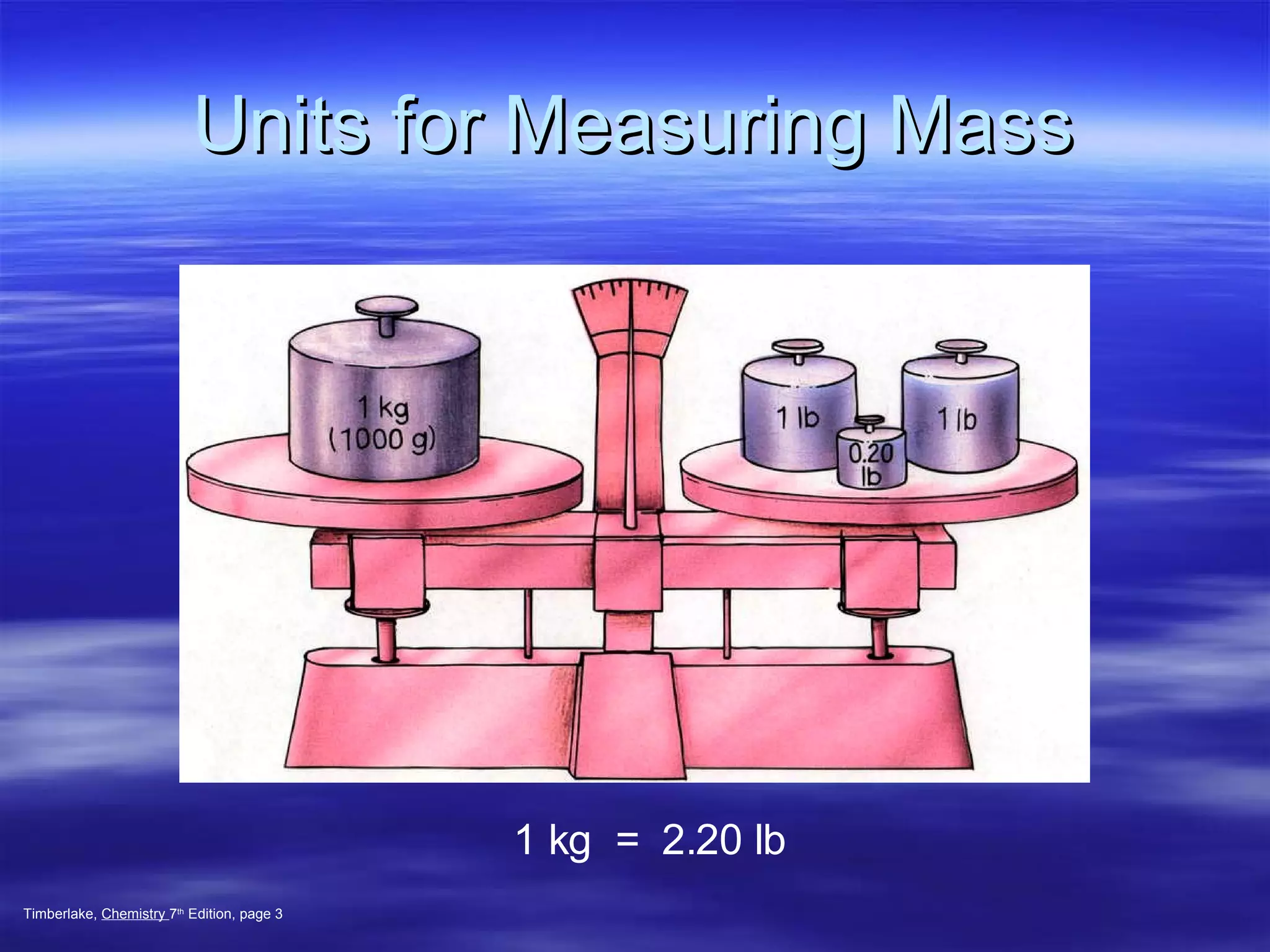
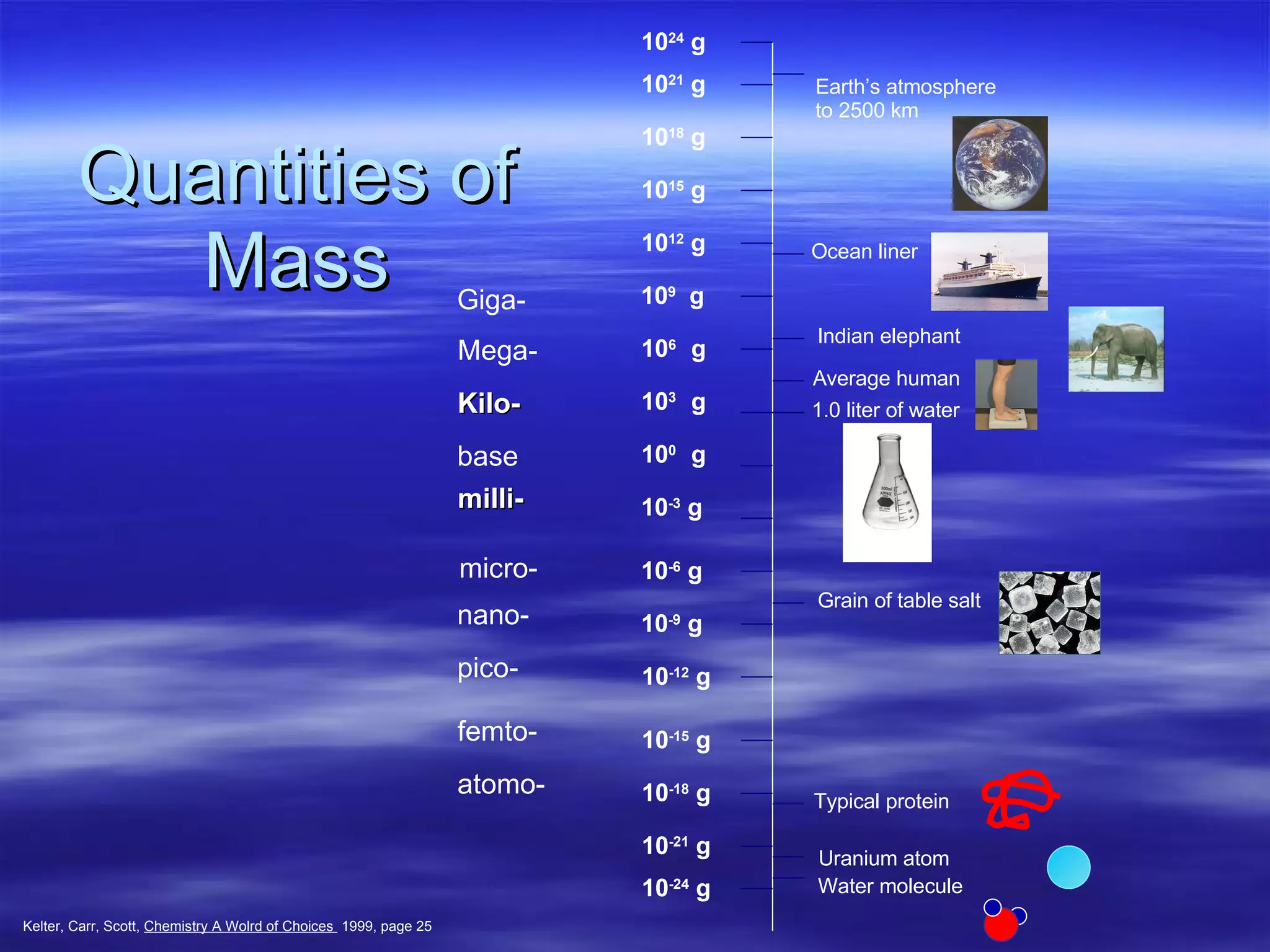
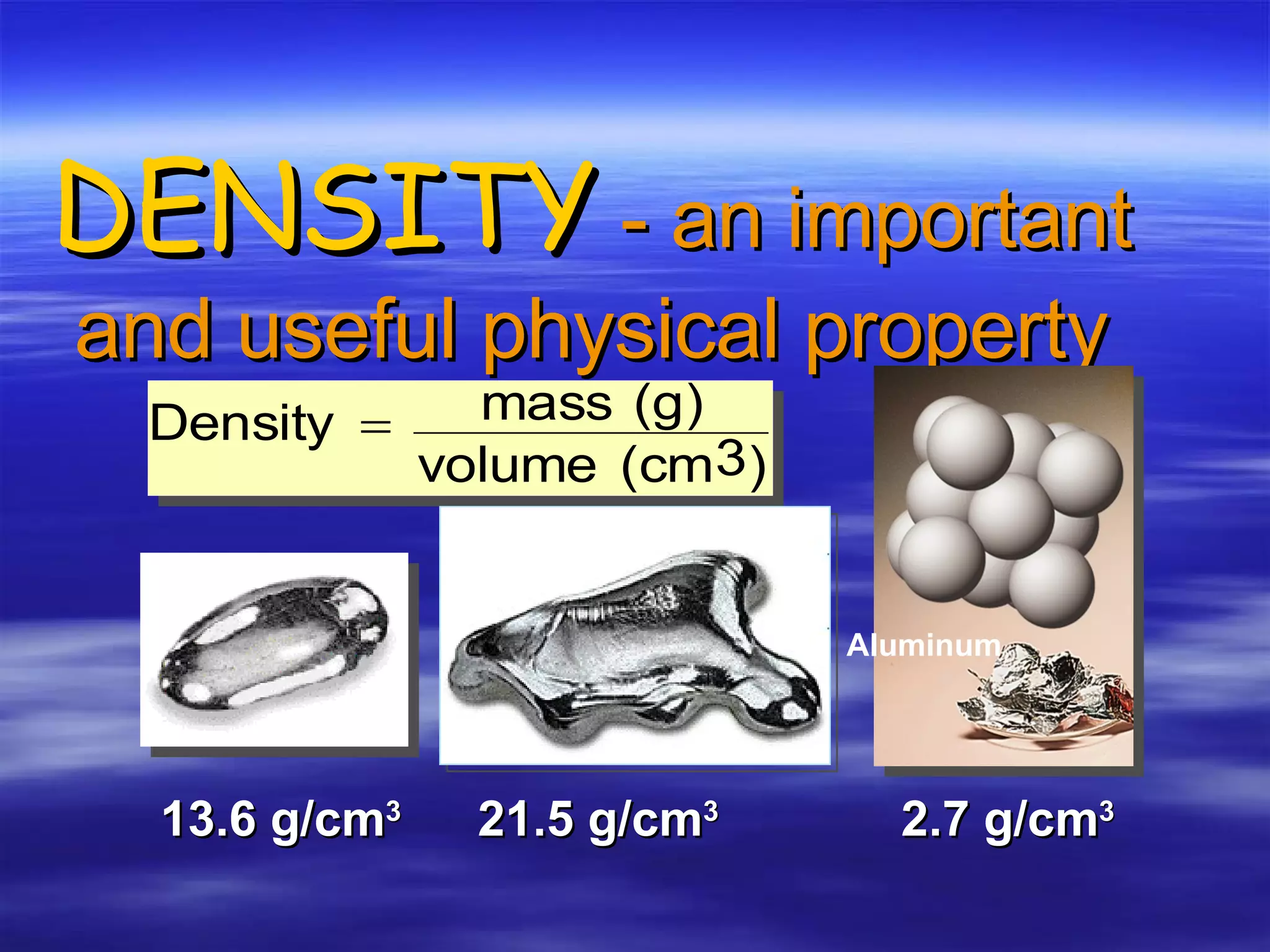
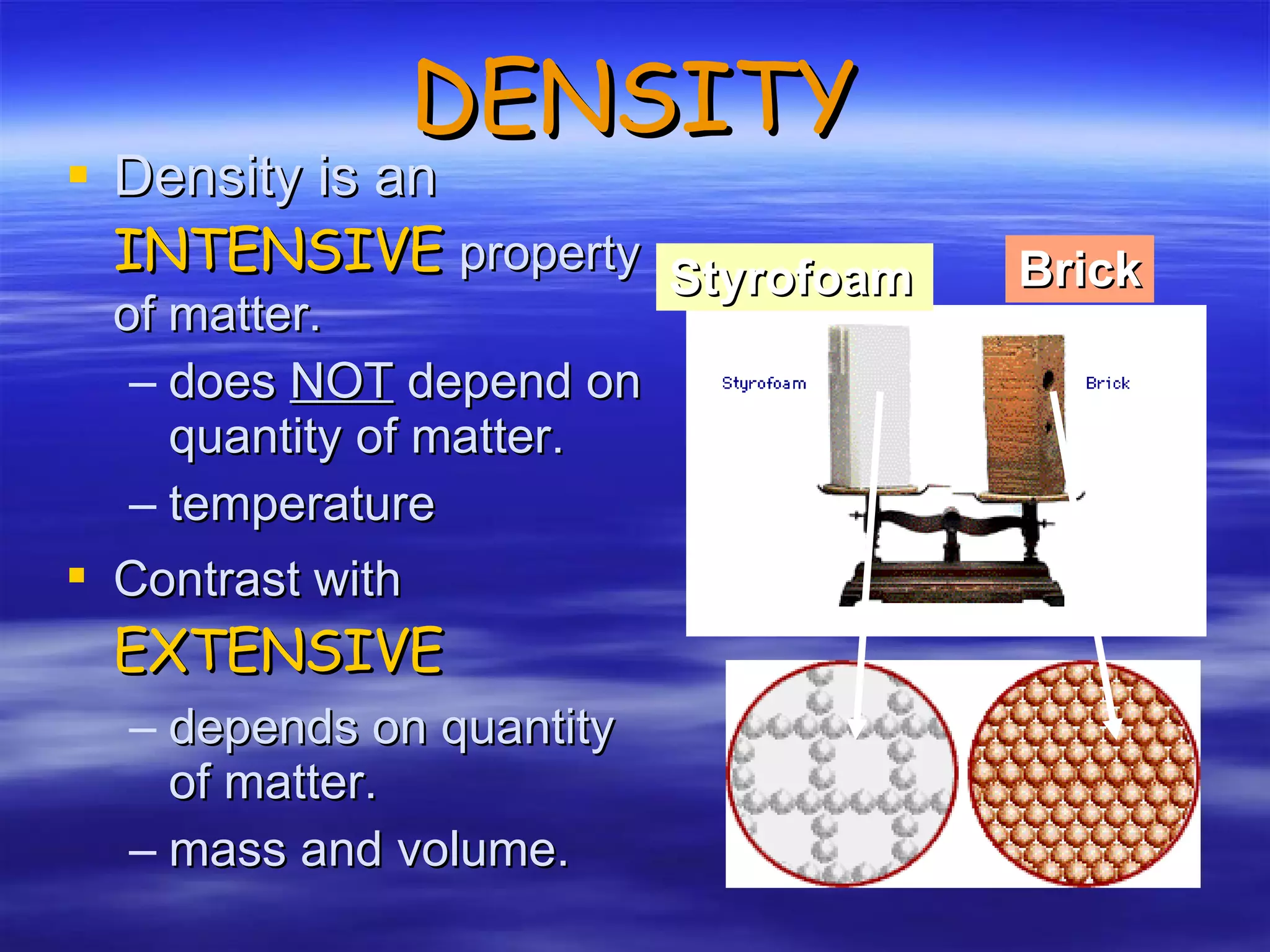
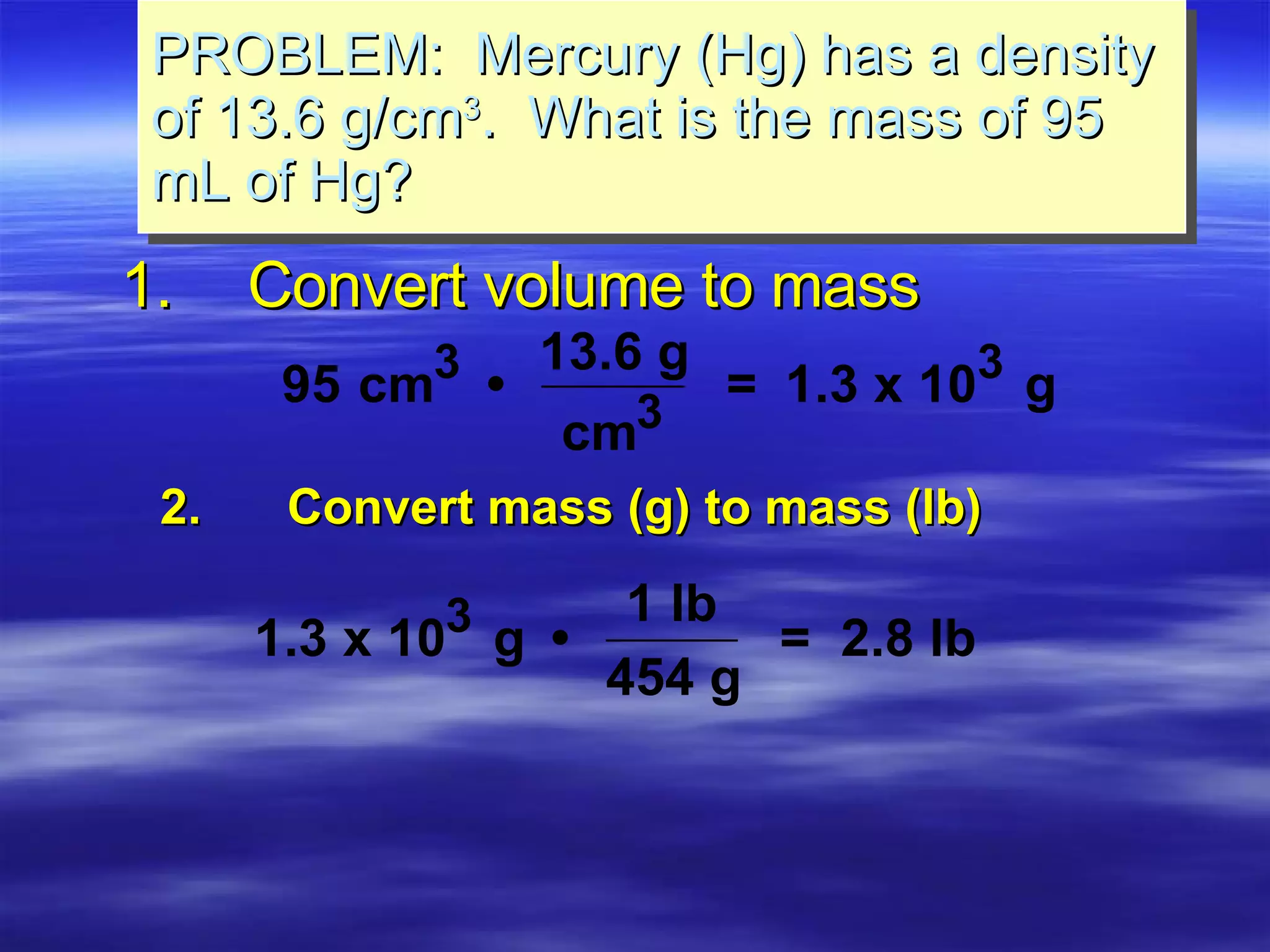
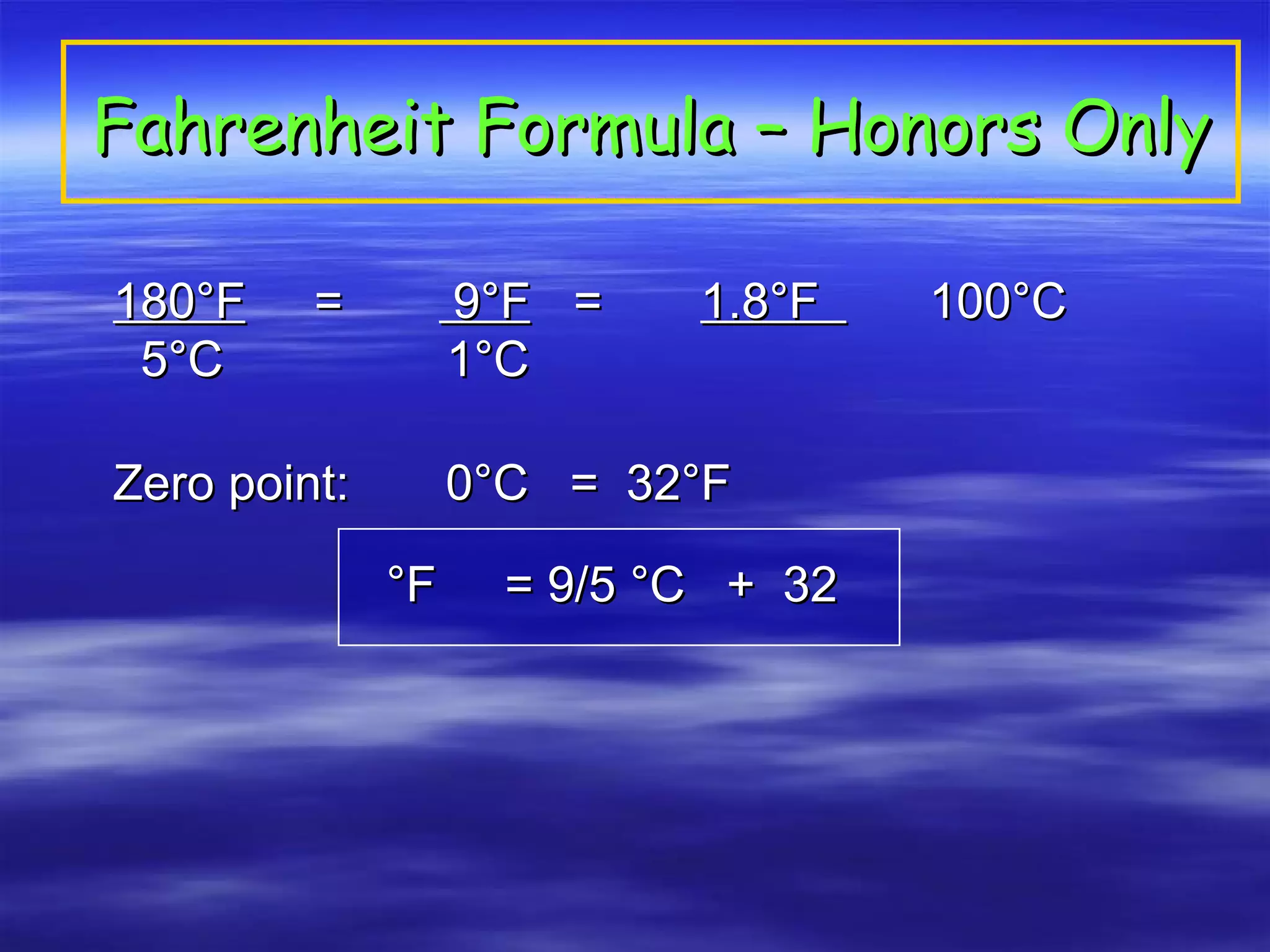
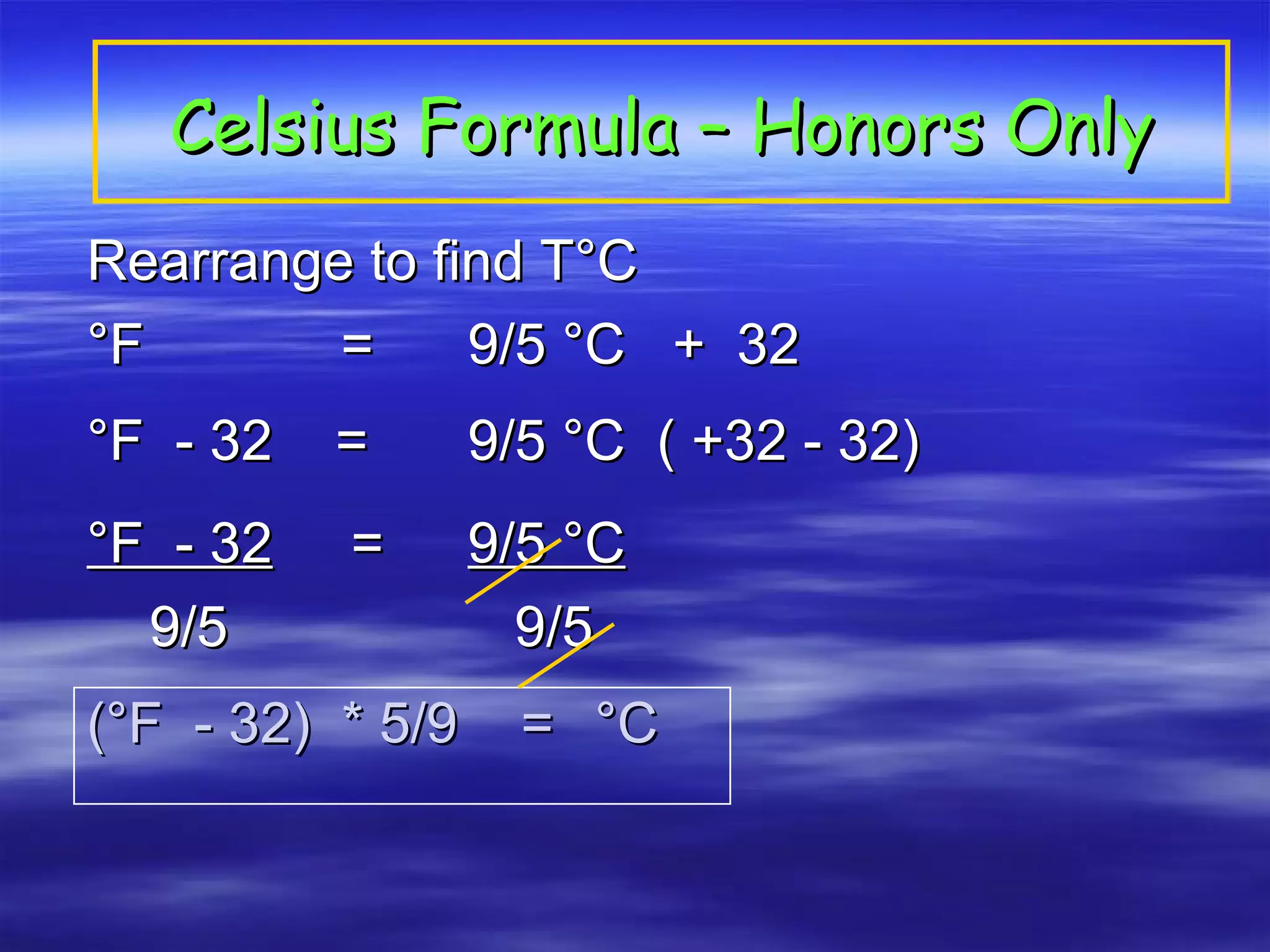
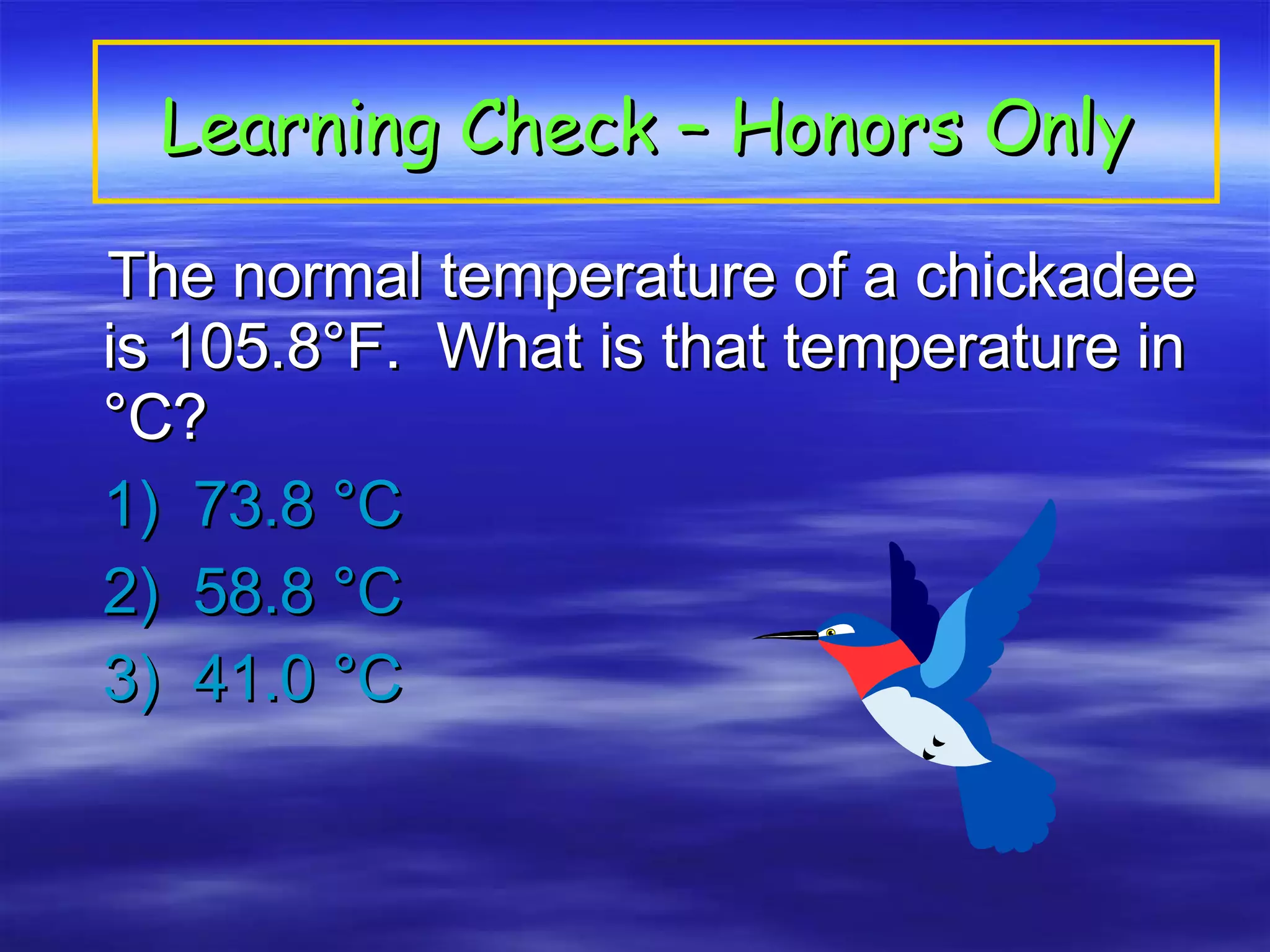
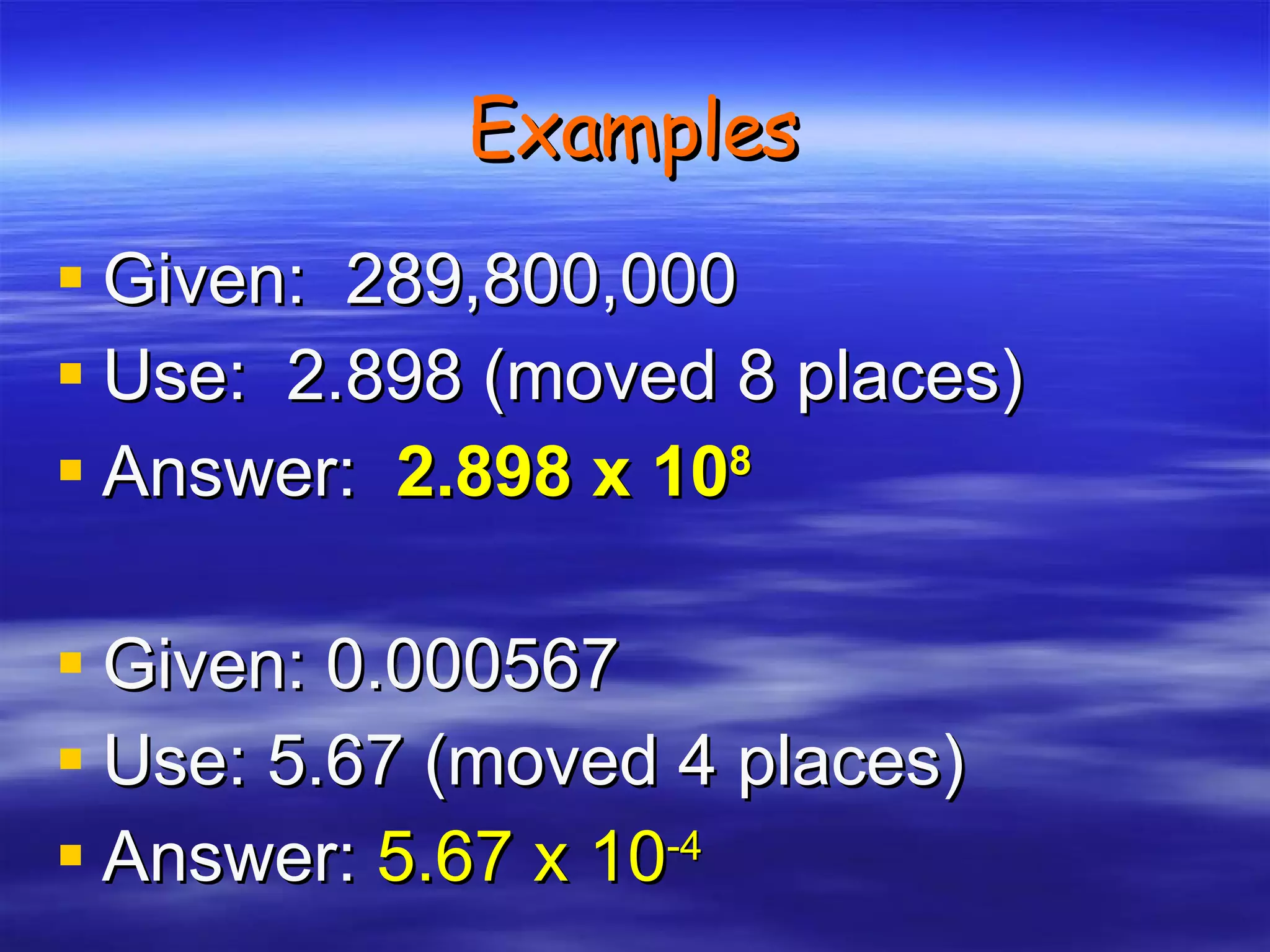
This document discusses measurement, density, and temperature scales. It defines qualitative and quantitative measurements. It explains scientific notation and how to convert between standard and scientific notation. It discusses accuracy versus precision. It describes metric units and prefixes for length, mass, volume, and temperature. It provides examples of calculating density and solving density problems using dimensional analysis. Finally, it introduces the Fahrenheit, Celsius, and Kelvin temperature scales.







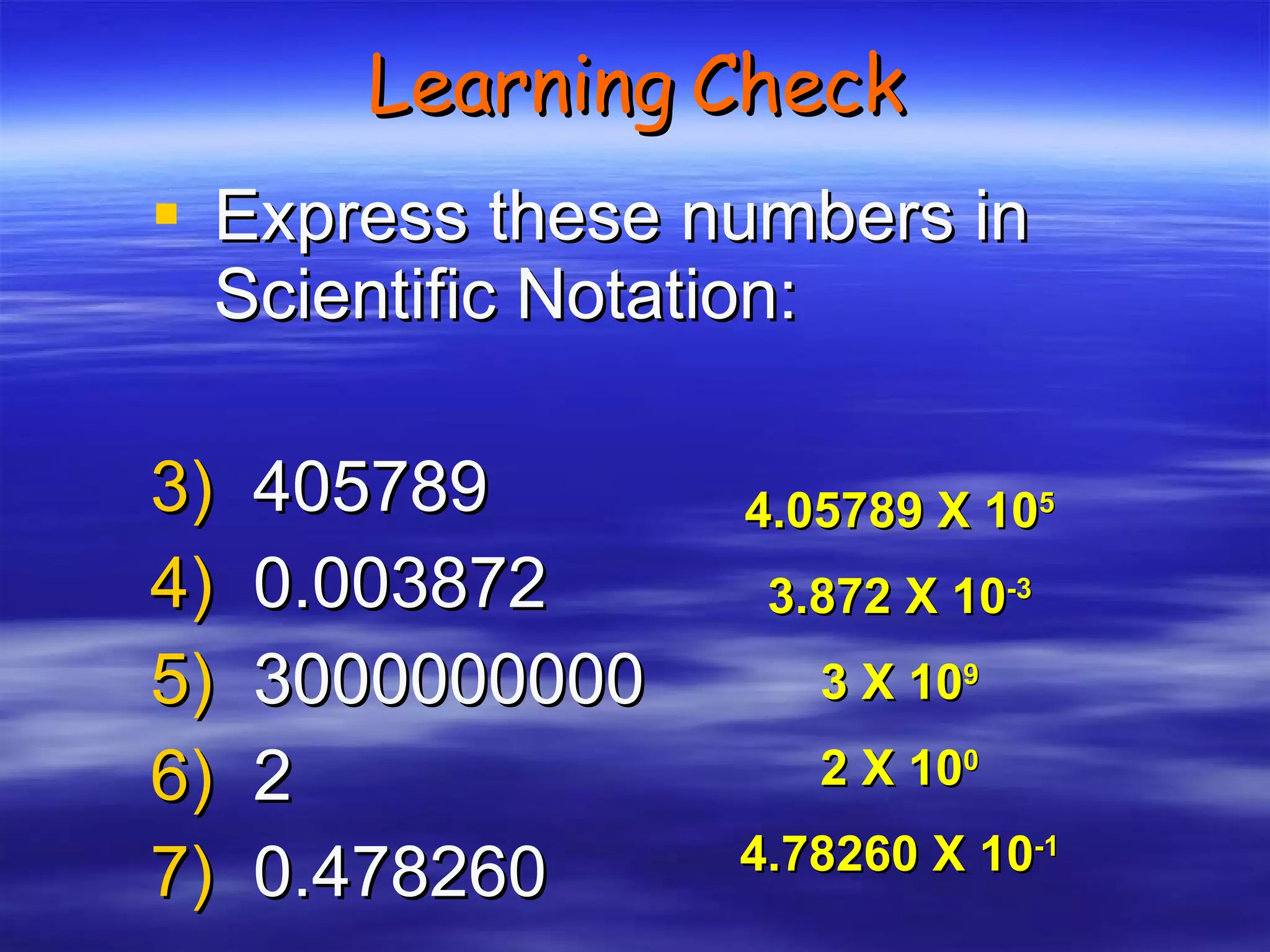



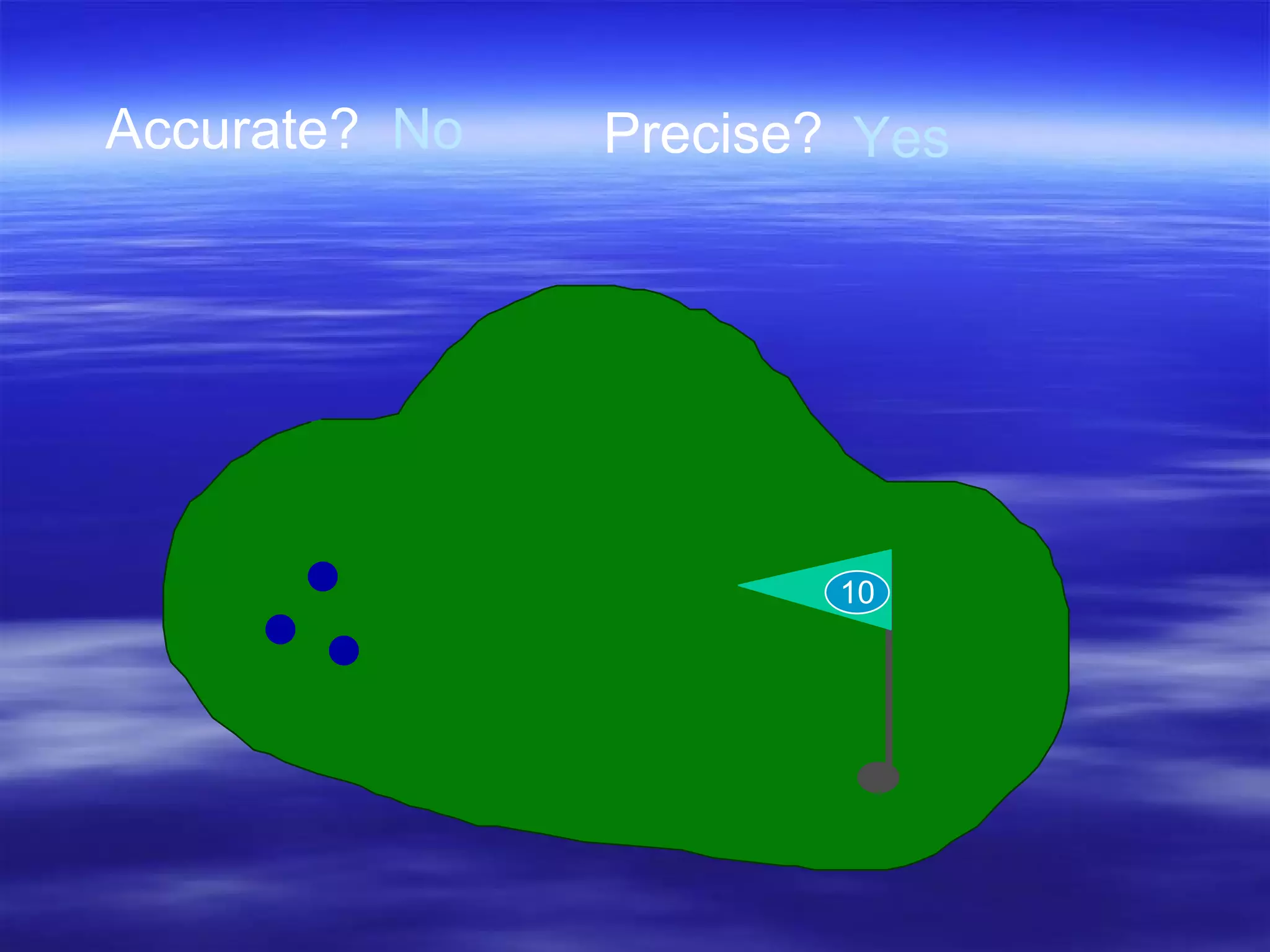
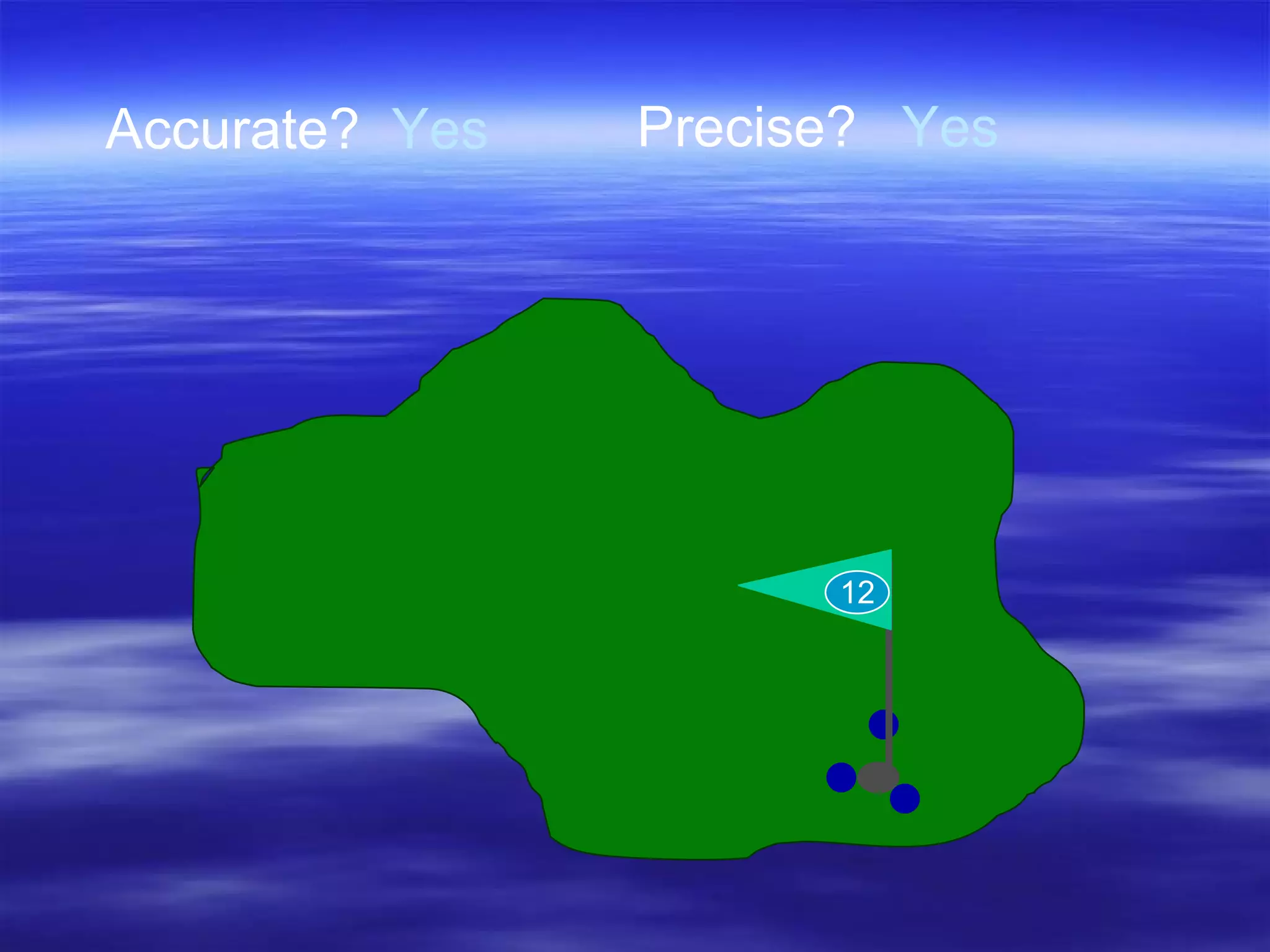
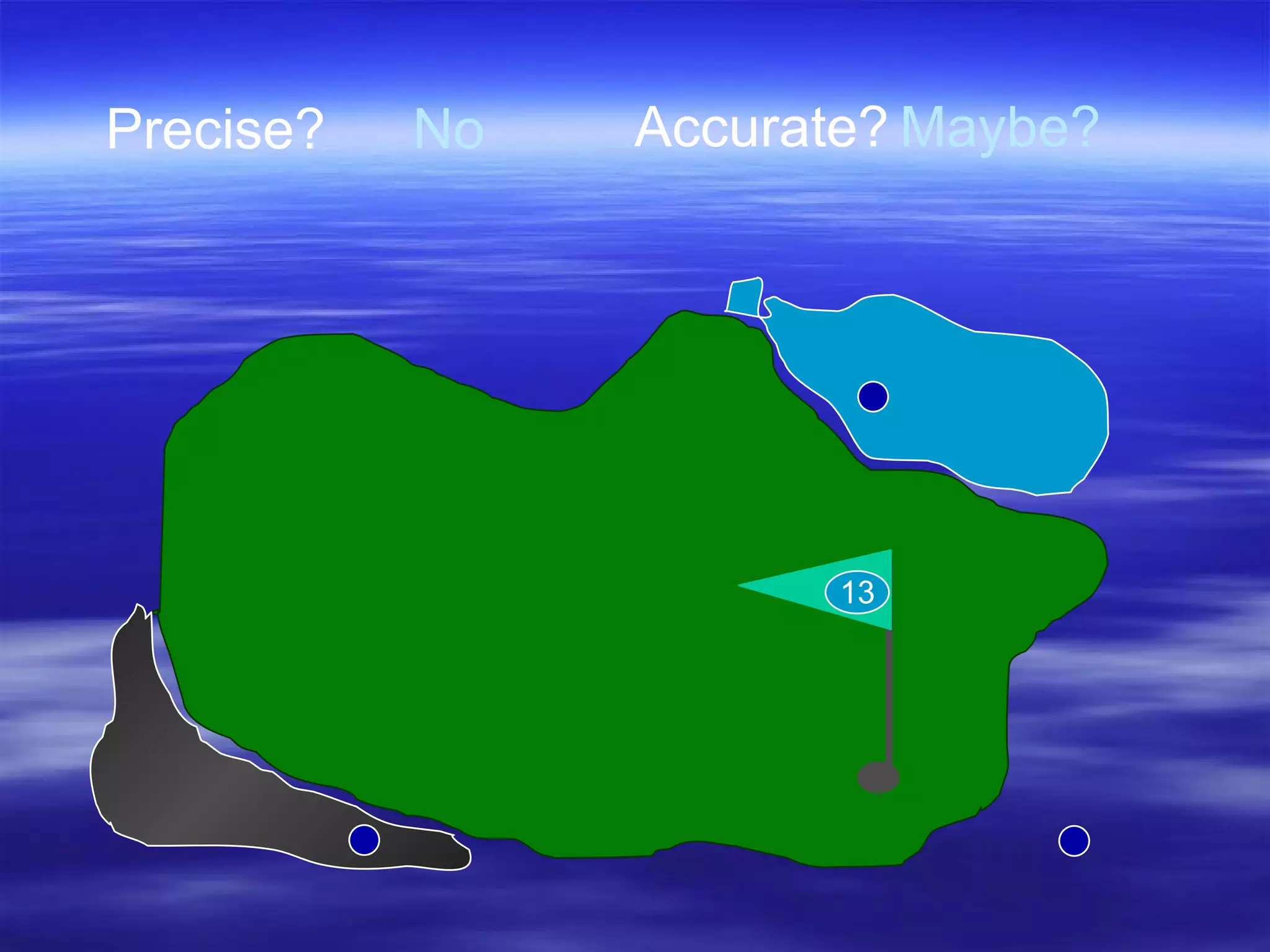



![Accuracy Precision Resolution subsequent samples time offset [arbitrary units] not accurate, not precise accurate, not precise not accurate, precise accurate and precise accurate, low resolution -2 -3 -1 0 1 2 3](https://image.slidesharecdn.com/ch-3-measurement-and-density-1212680133143084-8/75/Ch-3-Measurement-And-Density-19-2048.jpg)