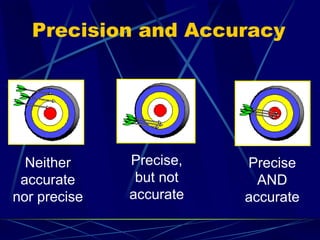
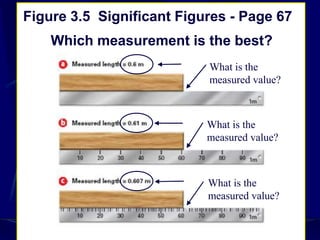
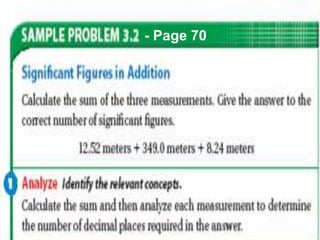
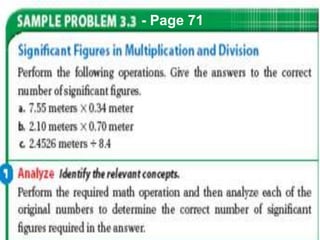
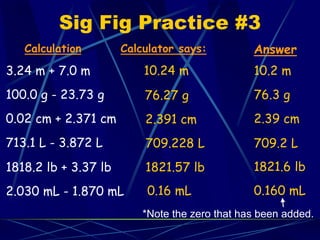
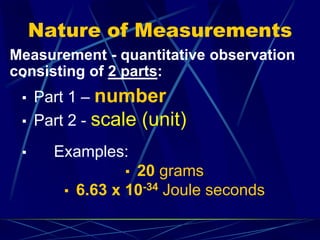
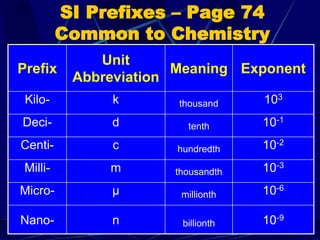
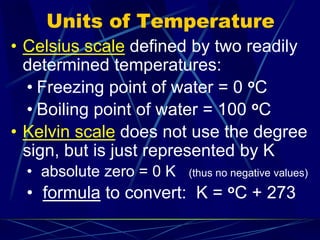
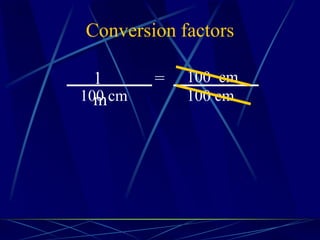
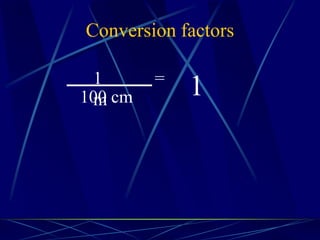
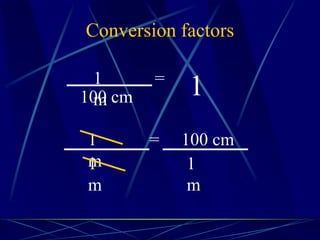
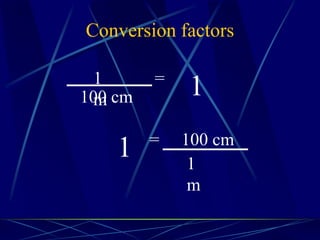
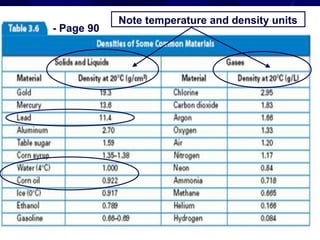
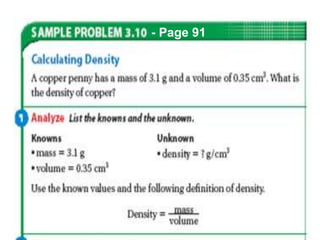
This document provides objectives and information about scientific measurement. It discusses accuracy, precision and error in measurements. It defines significant figures and how to determine them. It explains how to properly handle significant figures in calculations and addresses rounding. The document also discusses the International System of Units (SI) including base units like meters, kilograms and kelvin. It covers density, how it is calculated from mass and volume, and how density varies with temperature for most substances.