Mass spectrometry
•Download as PPTX, PDF•
1 like•248 views
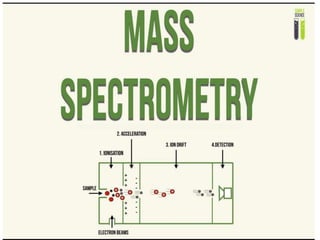
Mass spectrometry is a technique that measures the mass-to-charge ratio of ions. It works by ionizing analyte molecules, then separating the ions based on their m/z ratios using electric and magnetic fields. The data produced are mass spectra, which provide information about molecular structure. Key developments included techniques like electron ionization, electrospray ionization, and matrix-assisted laser desorption/ionization, which allowed analysis of nonvolatile and thermally labile compounds. Mass spectrometry determines isotopic composition and is used to study elemental, molecular, and isotopic properties of substances.
Report
Share
Report
Share
Recommended
instrumentation of mass spectrometry

This document provides an overview of the key components and operating principles of mass spectrometry. It discusses the inlet system, ion sources, mass analyzers, detectors, and vacuum system. Common types of ion sources like electron impact and chemical ionization are described. Popular mass analyzers such as quadrupole, time-of-flight, ion trap, and double focusing are explained. The document also covers the theory behind how mass spectrometry separates ions based on their mass-to-charge ratio and discusses the need for high vacuum levels in mass spectrometers.
Mass Spectroscopy 

Mass spectrometry is a technique that ionizes chemical species and sorts the ions based on their mass-to-charge ratio. It can be used to determine molecular masses and elucidate molecular structures of organic compounds. There are several types of ions produced including molecular ions, fragment ions, and isotope ions. Compounds undergo various fragmentation modes like homolytic cleavage, heterolytic cleavage, retro-Diels-Alder reactions, hydrogen transfers and McLafferty rearrangements. Mass spectrometry has applications in fields like drug development, environmental analysis, and clinical diagnosis.
TYPES OF POLAROGRAPHY.pptx

Polarography is a technique used for the qualitative and quantitative analysis of electro reducible or oxidized elements or groups. It is a electrochemical technique of analyzing solution that measure the current flowing between two electrodes in the solution as well as the gradually increasing applied voltage to determine respectively the concentration of solute and its nature.
Uv vis spectroscopy- part-1

UV-Visible spectroscopy involves absorption of ultraviolet or visible light by molecules. This absorption causes electrons to move to higher energy molecular orbitals. The Beer-Lambert law states that absorbance is directly proportional to concentration and path length. Deviations from Beer-Lambert law can occur at high concentrations due to interactions between molecules, or if non-monochromatic light is used. UV-Visible spectroscopy is used to study electronic transitions and structure of molecules.
NMR- Diamagnetic Anisotropy and its effect on chemical shift

The shift in the position of the NMR region resulting from the shielding and deshielding by electrons is called chemical shift.
When a proton is present inside the magnetic field more close to an electro positive atom more applied magnetic field is required to cause excitation. This effect is called shielding effect.
When a proton is present outside the magnetic field close to a electronegative atom less applied magnetic field is required to cause excitation . This effect is called deshielding effect
Ionization Techniques in Mass spectrometry.pptx

This document discusses various ionization techniques used in mass spectrometry. It describes the electron ionization process where a sample is ionized by high energy electrons in an ion source. The document outlines different ionization methods including gas phase sources like electron ionization and chemical ionization, as well as desorption sources like field ionization. It provides details on the electron ionization process, chemical ionization using various reagent gases, and field ionization which uses a strong electric field to ionize samples. Advantages and disadvantages of each technique are also summarized.
Infrared spectroscopy 

Infrared spectroscopy analyzes the absorption of infrared radiation by molecules to determine their structure. It works by exciting the vibrational modes of molecules, which correspond to characteristic absorption frequencies. The fingerprint region between 1500-500 cm-1 is especially useful for identifying functional groups and establishing molecular identity. Infrared spectrometers contain an infrared source, sample holder, detector, and recorder. Applications include identification of functional groups, structural elucidation of drugs and polymers, and quantitative analysis.
Flame emission spectroscopy

Flame photometry is a technique that uses the intensity of light emitted from a flame to determine the concentration of certain metal ions in a sample. When a sample is introduced into the flame, the metal ions are atomized and excited. As they return to the ground state, they emit light of characteristic wavelengths. The intensity of light emitted can then be measured to determine the concentration of the metal ions. Flame photometry is used to analyze samples for concentrations of ions like sodium, potassium, calcium, and lithium. It has applications in analyzing body fluids and determining metal concentrations in materials like cement.
Recommended
instrumentation of mass spectrometry

This document provides an overview of the key components and operating principles of mass spectrometry. It discusses the inlet system, ion sources, mass analyzers, detectors, and vacuum system. Common types of ion sources like electron impact and chemical ionization are described. Popular mass analyzers such as quadrupole, time-of-flight, ion trap, and double focusing are explained. The document also covers the theory behind how mass spectrometry separates ions based on their mass-to-charge ratio and discusses the need for high vacuum levels in mass spectrometers.
Mass Spectroscopy 

Mass spectrometry is a technique that ionizes chemical species and sorts the ions based on their mass-to-charge ratio. It can be used to determine molecular masses and elucidate molecular structures of organic compounds. There are several types of ions produced including molecular ions, fragment ions, and isotope ions. Compounds undergo various fragmentation modes like homolytic cleavage, heterolytic cleavage, retro-Diels-Alder reactions, hydrogen transfers and McLafferty rearrangements. Mass spectrometry has applications in fields like drug development, environmental analysis, and clinical diagnosis.
TYPES OF POLAROGRAPHY.pptx

Polarography is a technique used for the qualitative and quantitative analysis of electro reducible or oxidized elements or groups. It is a electrochemical technique of analyzing solution that measure the current flowing between two electrodes in the solution as well as the gradually increasing applied voltage to determine respectively the concentration of solute and its nature.
Uv vis spectroscopy- part-1

UV-Visible spectroscopy involves absorption of ultraviolet or visible light by molecules. This absorption causes electrons to move to higher energy molecular orbitals. The Beer-Lambert law states that absorbance is directly proportional to concentration and path length. Deviations from Beer-Lambert law can occur at high concentrations due to interactions between molecules, or if non-monochromatic light is used. UV-Visible spectroscopy is used to study electronic transitions and structure of molecules.
NMR- Diamagnetic Anisotropy and its effect on chemical shift

The shift in the position of the NMR region resulting from the shielding and deshielding by electrons is called chemical shift.
When a proton is present inside the magnetic field more close to an electro positive atom more applied magnetic field is required to cause excitation. This effect is called shielding effect.
When a proton is present outside the magnetic field close to a electronegative atom less applied magnetic field is required to cause excitation . This effect is called deshielding effect
Ionization Techniques in Mass spectrometry.pptx

This document discusses various ionization techniques used in mass spectrometry. It describes the electron ionization process where a sample is ionized by high energy electrons in an ion source. The document outlines different ionization methods including gas phase sources like electron ionization and chemical ionization, as well as desorption sources like field ionization. It provides details on the electron ionization process, chemical ionization using various reagent gases, and field ionization which uses a strong electric field to ionize samples. Advantages and disadvantages of each technique are also summarized.
Infrared spectroscopy 

Infrared spectroscopy analyzes the absorption of infrared radiation by molecules to determine their structure. It works by exciting the vibrational modes of molecules, which correspond to characteristic absorption frequencies. The fingerprint region between 1500-500 cm-1 is especially useful for identifying functional groups and establishing molecular identity. Infrared spectrometers contain an infrared source, sample holder, detector, and recorder. Applications include identification of functional groups, structural elucidation of drugs and polymers, and quantitative analysis.
Flame emission spectroscopy

Flame photometry is a technique that uses the intensity of light emitted from a flame to determine the concentration of certain metal ions in a sample. When a sample is introduced into the flame, the metal ions are atomized and excited. As they return to the ground state, they emit light of characteristic wavelengths. The intensity of light emitted can then be measured to determine the concentration of the metal ions. Flame photometry is used to analyze samples for concentrations of ions like sodium, potassium, calcium, and lithium. It has applications in analyzing body fluids and determining metal concentrations in materials like cement.
Mass spectrometry

Mass spectrometry is a powerful analytical technique that measures the mass-to-charge ratio of ions to identify molecules. It involves ionizing samples, separating the ions by their mass-to-charge ratio using electric or magnetic fields, and detecting the ions. Common applications include clinical analysis of metabolites and proteins. Key components include the ion source, which ionizes samples using techniques like electrospray ionization; the mass analyzer, such as quadrupoles, that separate ions; and the detector. Mass spectrometry provides qualitative and quantitative analysis of a wide range of clinically relevant compounds.
Ir spectroscopy slide

Infrared spectroscopy is technique to identify the functional group of the molecule.
In Infrared spectroscopy there are two main region finger print region and functional group region. Most of the molecules identifies In the finger print region due to that it is complex region.
Now we will see the
principle of IR spectroscopy:
IR spectroscopy is vibrational energy level changes when IR radiation passes through the material.
Deviations from Beers law

This document discusses various deviations from Beer's Law that can occur when using UV-Vis spectroscopy. There are three main types of deviations: 1) True deviations due to high analyte concentrations that cause interactions between molecules and changes in optical properties. 2) Instrumental deviations caused by factors like use of polychromatic light, improper slit width, stray light, mismatched cells, and scanning speed. 3) Chemical deviations due to shifts in chemical equilibria with changing concentration, like for pH indicators. Nonlinearity can be minimized by optimizing factors like concentration, slit width, and wavelength range used.
Capillary electrophoresis

Capillary electrophoresis is a separation technique that uses narrow bore capillaries. Charged molecules migrate through the capillary under the influence of an applied electric field and separate based on their charge and size. The principle involves electrostatic forces moving molecules toward the electrode of opposite charge, as well as electroosmotic flow dragging buffer molecules. Capillary electrophoresis has various modes of operation and is used to separate and analyze biological samples in clinical and diagnostic applications.
Sampling Techniques in IR Spectroscopy

Sampling Techniques in IR SpectroscopyRaghavendra institute of pharmaceutical education and research .
In this slide contains principle of IR spectroscopy and sampling techniques.
Presented by: R.Banuteja (Department of pharmaceutical analysis).
RIPER, anantpur.Mass spectrometry and ionization techniques

Mass spectrometry is a technique that identifies chemicals based on their mass and charge. It works by ionizing chemical compounds and separating the resulting ions based on their mass-to-charge ratio. The document discusses the key components and principles of mass spectrometry including various ionization methods, mass analyzers, and applications such as sequencing proteins, determining molecular weights, and drug discovery.
Chemical ionization

This document discusses mass spectrometry and chemical ionization. It explains that mass spectrometry can provide molecular structure information by determining molecular weight. Chemical ionization is a softer ionization technique than electron ionization, which causes less fragmentation and makes it easier to detect molecular ions. The document describes the instrumentation for chemical ionization, where a reagent gas like methane is ionized by electrons and the resulting ions collide with analyte molecules to form positively or negatively charged molecular ions depending on the mode used. The advantages of chemical ionization are also summarized.
Ms interpretation

The document discusses various fragmentation patterns seen in mass spectrometry for different functional groups. For alcohols, cleavage of the carbon-carbon bond adjacent to the oxygen is common, as is loss of a water molecule. Aromatic ethers often fragment through cleavage of the carbon-oxygen bond or rearrangement within the aromatic ring. Carboxylic acids may lose a hydroxyl group or carboxyl group through cleavage of bonds adjacent to the carbonyl. The presence of halides is indicated by isotopic peaks from chlorine or bromine atoms.
uv -visible spectroscopy

details about uv-visible spectroscopy. intoduction to uv-visible spectroscopy with principle,
instrumentation, application, beers lamberts law , detectors. helps to know details about uv-visible spectroscopy. complete notes of uv-visible spectroscopy.
Liquid chromatography–mass spectrometry (LC-MS) BY P. RAVISANKAR

Liquid chromatography–mass spectrometry (LC-MS) BY
P. RAVISANKAR
VIGNAN PHARMACY COLLEGE
VADLAMUDI
GUNTUR
A.P
INDIA
Ir spectroscopy ppt

Vibrational frequencies can shift from normal values due to several factors:
1) Coupled vibrations occur when bond vibrations interact, causing asymmetric and symmetric stretches at different frequencies than isolated bonds.
2) Fermi resonance involves coupling between fundamental and overtone vibrations, splitting peaks between the two modes.
3) Hydrogen bonding lowers frequencies as it strengthens interactions between donor and acceptor groups. Stronger bonding yields greater shifts to lower frequencies.
4) Electronic effects like induction, mesomerism, and field effects influence frequencies by strengthening or weakening bonds.
TYPES OF PEAKS IN MASS SPECTROSCOPY.pptx

Types of peaks in mass spectroscopy.
Molecular ion or parent peak.
base peak.
fragment ions.
rearrangement ion.
multiple charged ion.
negative ion.
metastable ion.
isotopes ion.
Infrared spectroscopy (I.R spectroscopy)

Infrared spectroscopy involves interaction of infrared radiation with matter. It covers absorption spectroscopy techniques and is conducted using an infrared spectrometer. The infrared region is divided into three regions based on wavelengths. Infrared spectroscopy follows Beer's law and analyzes the selective absorption bands in a sample's infrared spectrum to determine its molecular structure and identify functional groups, compounds, and impurities. It has applications in analyzing organic and inorganic compounds.
MASS SPECTROSCOPY

Mass spectrometry is a technique that ionizes chemical compounds and separates and identifies ions based on their mass-to-charge ratios. It can provide both qualitative and quantitative information about molecular structures. During analysis, molecules are bombarded by electrons which produces molecular ions and fragment ions. These ions are then separated by their mass in electric and magnetic fields. By analyzing the masses of molecular and fragment ions, mass spectrometry can determine molecular formulas and elucidate molecular structures. It is a highly sensitive technique that is widely used for molecular identification.
Relaxation in NMR 

A powerpoint presentation on relaxation in NMR and MRI, presented at MR journal club on 18 October 2006 in the Texas Medical Center.
Mass spectroscopy

Mass spectrometry is a technique used to identify molecules based on their mass. It works by ionizing chemical compounds to generate molecular or fragment ions and measuring their mass-to-charge ratios. The document discusses the basic principles and components of a mass spectrometer, including ionization, separation of ions based on mass, and detection. It also covers common fragmentation patterns observed for different classes of compounds like hydrocarbons, alcohols, aromatics, and others. General rules for fragmentation are provided along with examples to illustrate how structural information can be determined.
Nmr spectroscopy

1. 1H NMR spectroscopy is a technique used to analyze compounds by detecting hydrogen nuclei in a magnetic field. It provides information about functional groups, number of nuclei, and structure of compounds.
2. The principle involves hydrogen nuclei absorbing radio frequencies matching their Larmor frequency in an applied magnetic field. This absorption is measured to produce an NMR spectrum.
3. Factors like electronegativity, magnetic anisotropy, and spin-spin coupling influence the chemical shifts observed on the NMR spectrum, allowing identification of functional groups and structure elucidation.
NMR SPECTROSCOPY AND SOME PROBLEMS BASED ON IT

This presentation gives a brief overview on the theoretical aspects of NMR (proton & carbon), Instrumentation and some problems based on NMR
Mass spectrometry

1. Mass spectrometry is a technique used to measure the molecular weight and determine the molecular formula of an organic compound. It works by ionizing molecule samples and separating the resulting ions based on their mass-to-charge ratio.
2. In a mass spectrometer, a molecule sample is vaporized and bombarded with high-energy electrons, causing ionization through electron loss. The ions are then accelerated and deflected by electric or magnetic fields according to their mass.
3. A mass spectrum plots the relative abundance of each detected ion versus its mass-to-charge ratio. It provides information about molecular structure through identification of molecular and fragment ion peaks.
Introduction of mass spectrometer - basic types of mass analyzer 

The mass analyzer is the heart of the mass spectrometer, which takes ionized masses and separates them based on mass to charge ratios. There are several general types of mass analyzers, including magnetic sector, time of flight, quadrupole, ion trap
Mass Spectroscopy (Instrumentation & Spectral analysis ).pptx

Mass spectrometry is an analytical technique that identifies chemicals in a sample by measuring the mass-to-charge ratio of gas-phase ions. Samples are converted to rapidly moving positive ions via electron bombardment, then separated based on their mass-to-charge ratios. A mass spectrum plots relative abundance against mass-to-charge ratio and is used to determine elemental composition, molecular masses, and elucidate chemical structures. During analysis, molecules are ionized, accelerated, deflected according to their mass, and detected, providing information about a sample's composition.
Mass spectroscopy for M Sc I Chemistry SPPU

Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of ions. It involves converting the sample into gaseous ions, separating the ions based on their mass-to-charge ratio, and detecting the relative abundance of each ion. There are four key stages: ionization, acceleration, deflection according to mass-to-charge ratio, and detection. Different types of peaks in a mass spectrum provide information about the molecule, including the molecular ion peak, which indicates the molecular mass, and fragment ion peaks, which result from fragmentation of the molecular ion. Rules like the nitrogen rule and rule of 13 can help determine molecular formulas from mass spectrometry data.
More Related Content
What's hot
Mass spectrometry

Mass spectrometry is a powerful analytical technique that measures the mass-to-charge ratio of ions to identify molecules. It involves ionizing samples, separating the ions by their mass-to-charge ratio using electric or magnetic fields, and detecting the ions. Common applications include clinical analysis of metabolites and proteins. Key components include the ion source, which ionizes samples using techniques like electrospray ionization; the mass analyzer, such as quadrupoles, that separate ions; and the detector. Mass spectrometry provides qualitative and quantitative analysis of a wide range of clinically relevant compounds.
Ir spectroscopy slide

Infrared spectroscopy is technique to identify the functional group of the molecule.
In Infrared spectroscopy there are two main region finger print region and functional group region. Most of the molecules identifies In the finger print region due to that it is complex region.
Now we will see the
principle of IR spectroscopy:
IR spectroscopy is vibrational energy level changes when IR radiation passes through the material.
Deviations from Beers law

This document discusses various deviations from Beer's Law that can occur when using UV-Vis spectroscopy. There are three main types of deviations: 1) True deviations due to high analyte concentrations that cause interactions between molecules and changes in optical properties. 2) Instrumental deviations caused by factors like use of polychromatic light, improper slit width, stray light, mismatched cells, and scanning speed. 3) Chemical deviations due to shifts in chemical equilibria with changing concentration, like for pH indicators. Nonlinearity can be minimized by optimizing factors like concentration, slit width, and wavelength range used.
Capillary electrophoresis

Capillary electrophoresis is a separation technique that uses narrow bore capillaries. Charged molecules migrate through the capillary under the influence of an applied electric field and separate based on their charge and size. The principle involves electrostatic forces moving molecules toward the electrode of opposite charge, as well as electroosmotic flow dragging buffer molecules. Capillary electrophoresis has various modes of operation and is used to separate and analyze biological samples in clinical and diagnostic applications.
Sampling Techniques in IR Spectroscopy

Sampling Techniques in IR SpectroscopyRaghavendra institute of pharmaceutical education and research .
In this slide contains principle of IR spectroscopy and sampling techniques.
Presented by: R.Banuteja (Department of pharmaceutical analysis).
RIPER, anantpur.Mass spectrometry and ionization techniques

Mass spectrometry is a technique that identifies chemicals based on their mass and charge. It works by ionizing chemical compounds and separating the resulting ions based on their mass-to-charge ratio. The document discusses the key components and principles of mass spectrometry including various ionization methods, mass analyzers, and applications such as sequencing proteins, determining molecular weights, and drug discovery.
Chemical ionization

This document discusses mass spectrometry and chemical ionization. It explains that mass spectrometry can provide molecular structure information by determining molecular weight. Chemical ionization is a softer ionization technique than electron ionization, which causes less fragmentation and makes it easier to detect molecular ions. The document describes the instrumentation for chemical ionization, where a reagent gas like methane is ionized by electrons and the resulting ions collide with analyte molecules to form positively or negatively charged molecular ions depending on the mode used. The advantages of chemical ionization are also summarized.
Ms interpretation

The document discusses various fragmentation patterns seen in mass spectrometry for different functional groups. For alcohols, cleavage of the carbon-carbon bond adjacent to the oxygen is common, as is loss of a water molecule. Aromatic ethers often fragment through cleavage of the carbon-oxygen bond or rearrangement within the aromatic ring. Carboxylic acids may lose a hydroxyl group or carboxyl group through cleavage of bonds adjacent to the carbonyl. The presence of halides is indicated by isotopic peaks from chlorine or bromine atoms.
uv -visible spectroscopy

details about uv-visible spectroscopy. intoduction to uv-visible spectroscopy with principle,
instrumentation, application, beers lamberts law , detectors. helps to know details about uv-visible spectroscopy. complete notes of uv-visible spectroscopy.
Liquid chromatography–mass spectrometry (LC-MS) BY P. RAVISANKAR

Liquid chromatography–mass spectrometry (LC-MS) BY
P. RAVISANKAR
VIGNAN PHARMACY COLLEGE
VADLAMUDI
GUNTUR
A.P
INDIA
Ir spectroscopy ppt

Vibrational frequencies can shift from normal values due to several factors:
1) Coupled vibrations occur when bond vibrations interact, causing asymmetric and symmetric stretches at different frequencies than isolated bonds.
2) Fermi resonance involves coupling between fundamental and overtone vibrations, splitting peaks between the two modes.
3) Hydrogen bonding lowers frequencies as it strengthens interactions between donor and acceptor groups. Stronger bonding yields greater shifts to lower frequencies.
4) Electronic effects like induction, mesomerism, and field effects influence frequencies by strengthening or weakening bonds.
TYPES OF PEAKS IN MASS SPECTROSCOPY.pptx

Types of peaks in mass spectroscopy.
Molecular ion or parent peak.
base peak.
fragment ions.
rearrangement ion.
multiple charged ion.
negative ion.
metastable ion.
isotopes ion.
Infrared spectroscopy (I.R spectroscopy)

Infrared spectroscopy involves interaction of infrared radiation with matter. It covers absorption spectroscopy techniques and is conducted using an infrared spectrometer. The infrared region is divided into three regions based on wavelengths. Infrared spectroscopy follows Beer's law and analyzes the selective absorption bands in a sample's infrared spectrum to determine its molecular structure and identify functional groups, compounds, and impurities. It has applications in analyzing organic and inorganic compounds.
MASS SPECTROSCOPY

Mass spectrometry is a technique that ionizes chemical compounds and separates and identifies ions based on their mass-to-charge ratios. It can provide both qualitative and quantitative information about molecular structures. During analysis, molecules are bombarded by electrons which produces molecular ions and fragment ions. These ions are then separated by their mass in electric and magnetic fields. By analyzing the masses of molecular and fragment ions, mass spectrometry can determine molecular formulas and elucidate molecular structures. It is a highly sensitive technique that is widely used for molecular identification.
Relaxation in NMR 

A powerpoint presentation on relaxation in NMR and MRI, presented at MR journal club on 18 October 2006 in the Texas Medical Center.
Mass spectroscopy

Mass spectrometry is a technique used to identify molecules based on their mass. It works by ionizing chemical compounds to generate molecular or fragment ions and measuring their mass-to-charge ratios. The document discusses the basic principles and components of a mass spectrometer, including ionization, separation of ions based on mass, and detection. It also covers common fragmentation patterns observed for different classes of compounds like hydrocarbons, alcohols, aromatics, and others. General rules for fragmentation are provided along with examples to illustrate how structural information can be determined.
Nmr spectroscopy

1. 1H NMR spectroscopy is a technique used to analyze compounds by detecting hydrogen nuclei in a magnetic field. It provides information about functional groups, number of nuclei, and structure of compounds.
2. The principle involves hydrogen nuclei absorbing radio frequencies matching their Larmor frequency in an applied magnetic field. This absorption is measured to produce an NMR spectrum.
3. Factors like electronegativity, magnetic anisotropy, and spin-spin coupling influence the chemical shifts observed on the NMR spectrum, allowing identification of functional groups and structure elucidation.
NMR SPECTROSCOPY AND SOME PROBLEMS BASED ON IT

This presentation gives a brief overview on the theoretical aspects of NMR (proton & carbon), Instrumentation and some problems based on NMR
Mass spectrometry

1. Mass spectrometry is a technique used to measure the molecular weight and determine the molecular formula of an organic compound. It works by ionizing molecule samples and separating the resulting ions based on their mass-to-charge ratio.
2. In a mass spectrometer, a molecule sample is vaporized and bombarded with high-energy electrons, causing ionization through electron loss. The ions are then accelerated and deflected by electric or magnetic fields according to their mass.
3. A mass spectrum plots the relative abundance of each detected ion versus its mass-to-charge ratio. It provides information about molecular structure through identification of molecular and fragment ion peaks.
Introduction of mass spectrometer - basic types of mass analyzer 

The mass analyzer is the heart of the mass spectrometer, which takes ionized masses and separates them based on mass to charge ratios. There are several general types of mass analyzers, including magnetic sector, time of flight, quadrupole, ion trap
What's hot (20)
Liquid chromatography–mass spectrometry (LC-MS) BY P. RAVISANKAR

Liquid chromatography–mass spectrometry (LC-MS) BY P. RAVISANKAR
Introduction of mass spectrometer - basic types of mass analyzer 

Introduction of mass spectrometer - basic types of mass analyzer
Similar to Mass spectrometry
Mass Spectroscopy (Instrumentation & Spectral analysis ).pptx

Mass spectrometry is an analytical technique that identifies chemicals in a sample by measuring the mass-to-charge ratio of gas-phase ions. Samples are converted to rapidly moving positive ions via electron bombardment, then separated based on their mass-to-charge ratios. A mass spectrum plots relative abundance against mass-to-charge ratio and is used to determine elemental composition, molecular masses, and elucidate chemical structures. During analysis, molecules are ionized, accelerated, deflected according to their mass, and detected, providing information about a sample's composition.
Mass spectroscopy for M Sc I Chemistry SPPU

Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of ions. It involves converting the sample into gaseous ions, separating the ions based on their mass-to-charge ratio, and detecting the relative abundance of each ion. There are four key stages: ionization, acceleration, deflection according to mass-to-charge ratio, and detection. Different types of peaks in a mass spectrum provide information about the molecule, including the molecular ion peak, which indicates the molecular mass, and fragment ion peaks, which result from fragmentation of the molecular ion. Rules like the nitrogen rule and rule of 13 can help determine molecular formulas from mass spectrometry data.
Mass spectrometry

Mass spectrometry is an analytical technique that identifies chemicals in a sample by measuring the mass-to-charge ratio of ions. Samples are converted to ions, typically by bombarding them with electrons, and the ions are then separated by their mass and detected. A mass spectrum plots the relative abundance of detected ions against their mass-to-charge ratio. Mass spectrometry is used to determine molecular structures and elucidate chemical compositions of molecules and compounds. It has applications in environmental monitoring, chemical analysis, proteomics, metabolomics, and more.
Mass spectroscopy for MSc I Chemistry of SPPU

* Using Rule of 13:
* Molecular mass = 128
* 128/13 = 9 with remainder of 8
* So the hydrocarbon formula is C9H9+8 = C9H17
* It is given that there are 8 hydrogens
* So remove 8 hydrogens from C9H17 to get C9H9
Therefore, the molecular formula is C9H9
Mass Spectrometry

Mass spectrometry is an analytical technique that identifies unknown compounds and quantifies known materials by measuring their mass-to-charge ratios. It works by ionizing chemical compounds, generating charged molecule fragments, and measuring their mass-to-charge ratios using techniques like time-of-flight analysis. The document discusses the principles, instrumentation including ion sources, mass analyzers, and detectors, applications in fields like proteomics and metabolomics, and guidelines for interpreting mass spectra.
Mass spectrometry basic principle & Instrumentation

Mass spectrometry is an analytical technique that identifies chemicals in a sample by measuring the mass-to-charge ratio and abundance of gas-phase ions. It works by bombarding molecule samples with electrons to produce positively charged ions, which are then separated by mass and detected. Mass spectra plots show the relative abundance of ions and are used to determine molecular structure and composition.
Bilal Ibrahim Dan-Iya_GS57595.pptx

1. Mass spectrometry is an analytical technique that identifies compounds by measuring their mass-to-charge ratio and abundance. It works by converting molecules to ions, and characterizing them based on their mass and relative abundance.
2. Key applications of mass spectrometry include proteomics, drug discovery, clinical testing, genomics, and environmental analysis.
3. Common mass spectrometry techniques involve ionizing samples, separating the ions using electric or magnetic fields, and detecting the ions. This document focuses on the contributions of scientists to developing mass spectrometry and its applications in proteomics research.
Stable isotops ratio mass spectrometry

IRMS and AMS are specialized mass spectrometry techniques. [IRMS] determines the relative abundances of isotopes in a sample to provide information about its geographic, chemical, and biological origins. [AMS] accelerates ions to extremely high energies before mass analysis, allowing it to separate rare isotopes from abundant ones. Both techniques involve ionizing the sample, separating the ions by mass/charge ratio using magnetic and electric fields, and detecting the relative abundances of isotopes.
Isotopes and Radioactive Decat

This document discusses isotopes and radioactive decay. It defines isotopes as forms of a chemical element with the same number of protons but different numbers of neutrons. It describes the history of discoveries in radioactivity by Henri Becquerel, Frederick Soddy, and Ernest Rutherford. It discusses the types of radioactive particles (alpha, beta, gamma rays), their properties, and how radioactive decay occurs. It also covers radioactive decay rates, units of radioactivity like curies, and the concept of half-life.
Atomic Theory.pptx

Rutherford's gold foil experiment involved firing alpha particles at a thin sheet of gold foil and observing their scattering. The key findings were:
- The nucleus is small and dense
- It is positively charged
- Most alpha particles passed through the foil with little deflection, but a few were greatly deflected, surprising Rutherford.
This led to the conclusion that atoms have a small, dense, positively charged nucleus surrounded by orbiting electrons. The modern atomic model consists of protons and neutrons in the nucleus surrounded by electrons in an electron cloud.
Mass spectrometry- full lecture 

Mass spectrometry is an analytical technique that ionizes molecular fragments and sorts the subsequent ions based on their mass-to-charge ratio. It can be used to determine molecular mass and structure. The document discusses the basic principles, instrumentation, and fragmentation patterns of mass spectrometry. The instrumentation involves an ion source, mass analyzer, and detector. Molecular ions, fragment ions, and rearrangement ions are produced through electron bombardment. Fragmentation occurs primarily through homolytic or heterolytic cleavage and can produce stable carbocations. Mass spectrometry provides molecular weight and structural information.
Mass.pptx instrumentation, principle, theory

Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of ions to identify unknown compounds and determine molecular structure. It works by ionizing atom or molecules and then separating the resulting ions based on their mass-to-charge ratio to produce a mass spectrum. There are various ionization methods used including hard ionization techniques like electron impact that cause fragmentation and soft techniques like electrospray ionization and matrix-assisted laser desorption/ionization that produce little fragmentation. Mass spectrometry has wide applications in fields like environmental analysis, forensics, pharmaceutical analysis, and more.
mass spectrocopy (khushi).pdf

This document summarizes a seminar presentation on mass spectrometry. It introduces mass spectrometry and its basic principles, instrumentation, ionization techniques, types of ions, fragmentation rules like McLafferty rearrangement. It then discusses applications in environmental monitoring, geochemistry, chemical industry, and biomolecule identification.
Mass spectrometry 

This document provides an overview of mass spectrometry. It defines mass spectrometry as a technique for determining the molecular mass and composition of organic and inorganic compounds. The document outlines the basic components and working principle of a mass spectrometer. It discusses sample ionization, mass analysis, and ion detection. Fragmentation patterns are described along with examples of mass spectra for different compound classes like alkanes, alkenes, alkynes, aromatics, and alcohols. The history of mass spectrometry is also summarized.
New microsoft power point presentation1 new

Mass spectrometry is a technique that involves ionizing molecules and analyzing their mass-to-charge ratios. It works by generating molecular or fragment ions from a sample, separating them based on their mass-to-charge ratio, and detecting the relative abundance of each ion. Different types of mass spectrometers use various ionization methods like electron impact, electrospray, or matrix-assisted laser desorption to ionize samples. Mass spectrometry is used to determine molecular masses and identify unknown substances based on their fragmentation patterns and isotopic abundances.
Mass spectrometry.pptx

Mass spectrometry is a technique that identifies unknown substances based on the mass-to-charge ratio of ions. It works by ionizing analyte molecules and separating the resulting ions in an electric or magnetic field based on their m/z ratio. Different ionization techniques like electron ionization, chemical ionization, electrospray ionization, and matrix-assisted laser desorption/ionization are used depending on the sample's physical state and properties. Mass analyzers like quadrupoles and time-of-flight instruments then measure the m/z of ionized molecules to generate mass spectra for analysis.
Basic concepts in chemistry

John Dalton proposed the first scientific atomic theory, which stated that each chemical element is composed of atoms of a single, unique type that can combine to form chemical compounds. An atom is the fundamental unit of matter and consists of a nucleus containing protons and neutrons surrounded by electrons. Atoms of the same element contain the same number of protons but can vary in the number of neutrons, resulting in different isotopes of that element. Molecules are the smallest fundamental units of compounds made of two or more atoms bonded together.
Mass spectrometry

Mass spectrometry is a technique that analyzes molecules by generating ionized molecules and measuring their mass-to-charge ratios. A mass spectrometer bombards neutral molecules with electrons, producing molecular ions and fragments that are separated by their mass-to-charge ratios and detected. The resulting mass spectrum plots the relative abundances of ions versus mass-to-charge ratios and provides information about molecular weight, structure, and isotopic composition. Interpreting mass spectra involves identifying peaks corresponding to molecular ions and common isotopes to deduce the molecular formula.
Mass analyse rs by bashant

This document provides an overview of mass spectrometry. It discusses the basic principles, including how molecules are ionized by bombarding them with electrons, and how the ions are then separated based on their mass-to-charge ratio using different types of analyzers like quadrupole, time-of-flight, and magnetic sector analyzers. It also describes how each analyzer works and its advantages and limitations. The document emphasizes that mass spectrometry is useful for determining molecular structure and identifying unknown compounds.
Similar to Mass spectrometry (20)
Mass Spectroscopy (Instrumentation & Spectral analysis ).pptx

Mass Spectroscopy (Instrumentation & Spectral analysis ).pptx
Mass spectrometry basic principle & Instrumentation

Mass spectrometry basic principle & Instrumentation
More from gopinathannsriramachandraeduin
Air pollution

Air pollution has existed for centuries, exacerbated by the industrial revolution and rise of automobiles. Some key events in air pollution history include Hippocrates mentioning it in 400 BC, London's Smog of 1952 killing over 4,000, and the Bhopal gas tragedy of 1984 in India. The atmosphere is composed mainly of nitrogen and oxygen, and is divided into layers including the troposphere containing most air pollution. Primary pollutants such as carbon monoxides, nitrogen oxides, and particulates are emitted directly, while secondary pollutants form through atmospheric reactions. Unchecked air pollution can have severe health and environmental impacts.
What happens to pollutants in the atmosphere

Once pollutants enter the atmosphere, they are transported by wind and diluted by air volumes. Some are removed by precipitation. On still evenings, cold air traps near the ground can cause fog or smog as pollutants accumulate. Strong winds disperse pollutants faster, while stagnant air allows higher concentrations. Air pollutants form acid rain when transported by wind and can damage ecosystems, structures, and human health. The ozone layer protects from UV rays but is depleted by CFCs, increasing UV exposure risks.
Water pollution

Water is essential for life on Earth but only a tiny fraction of water is available as freshwater for human use. While oceans hold 97% of the planet's water, only 0.003% is easily accessible as freshwater in lakes, rivers, soil and aquifers. Groundwater aquifers are an important source of freshwater but are vulnerable to pollution from agricultural, industrial and urban activities that can contaminate drinking water sources. Many forms of water pollution including disease-causing pathogens, oxygen-depleting wastes, excess nutrients, toxic chemicals, and radioactive substances threaten the limited freshwater resources and ecosystems upon which all life depends. Strategies are needed to protect this vital resource from overuse and pollution.
Solid waste management

Municipal solid waste management has evolved significantly over time. Early cities simply threw waste into streets, while now collection, processing, recycling and disposal are integrated. Sources of municipal solid waste include homes, businesses and some industrial sites, excluding hazardous, construction, sewage or agricultural waste. Waste is classified as wet (food) or dry (plastics, paper). Management strategies include reducing waste, recycling materials like metal and glass, and disposing of remaining waste through sanitary landfills or incineration. Vermicomposting is a natural alternative that uses worms to break down organic waste into nutrient-rich soil.
Soil pollution

The document discusses soil formation and degradation. It states that soil is formed over long periods from the weathering of rock by various physical, chemical, and biological processes. Climate and time influence soil development, with warmer, wetter climates producing soil more rapidly. Mature soil consists of distinct horizontal layers called horizons. The document outlines some key causes of soil degradation, including erosion from wind and water; loss of nutrients due to harvesting without replenishing fertilizers; and salinization from irrigation in arid areas. Prevention of soil erosion involves techniques such as planting vegetation, using mulch, improving drainage, and reducing over-watering.
Nuclear hazards

The document discusses various aspects of nuclear energy and hazards, including:
- Nuclear energy can be beneficial when used for applications like medicine but also causes environmental damage from radioactive waste.
- Major nuclear disasters like Chernobyl caused widespread contamination and health issues due to radiation exposure from the reactor explosion.
- Long-term storage and disposal of radioactive nuclear waste is challenging due to the waste's long half-lives and potential for contamination if not properly isolated from the environment for thousands of years. Methods under consideration include geological disposal, reprocessing, transmutation, and space disposal.
Noise pollution

Noise pollution can negatively impact human health and the environment. It is caused by loud sounds from sources like vehicles, construction sites, and industrial activity. Prolonged exposure to noise above safe levels can lead to hearing loss, cardiovascular issues, and disrupted sleep. Methods to reduce noise pollution include using barriers around noisy machinery, limiting vehicle speeds, planting trees as buffers, and protecting workers' hearing with earplugs. Controlling noise at the source is the most effective approach.
Marine pollution

Marine pollution is caused by human activities that introduce waste into the ocean directly or indirectly. Waste enters the ocean through pipes discharging municipal waste and sewage, agricultural runoff of pesticides and fertilizers, petroleum and oils washed from roads into waterways, offshore oil extraction and accidents that release toxic substances from ships. This pollution reduces oxygen levels in the water, releases toxic compounds, and harms marine life through effects like clogging gills or reducing birds' ability to regulate body temperature. Treatment plants can help by removing waste and reducing pollution load through primary, secondary, and advanced treatment methods.
Structure of atmosphere

The atmosphere is composed of several distinct layers. The innermost layer is the troposphere, which extends from the Earth's surface to around 17 km high at the equator. It contains around 75% of the atmosphere's mass. Above the troposphere is the stratosphere, which extends from 17-48 km high. It contains higher levels of ozone which absorbs over 99% of the sun's harmful UV radiation. The stratosphere is followed by the mesosphere and thermosphere, with temperatures decreasing and then increasing with altitude.
Five primary pollutants air pollution

The document discusses the primary pollutants that contribute to 90% of global air pollution, including carbon oxides, nitrogen oxides, sulfur oxides, volatile organic compounds, and particulate matter. It also discusses secondary pollutants that are produced through chemical reactions between primary pollutants like sulfuric acid and nitric acid. Specific primary pollutants like carbon monoxide from incomplete combustion, sulfur oxides from burning fossil fuels, and nitrogen oxides from vehicle exhaust are examined in more detail.
Air pollution

Air pollution has been recognized as a problem for centuries. King Edward I made the first antipollution law in 1273 to restrict coal burning in London due to smoke pollution. The deadliest event was the 1952 London Smog that resulted in over 4,000 deaths over 5 days due to accumulated pollutants. Air pollution increased in the 20th century with the rise of transportation systems using petrol and diesel. The Bhopal gas tragedy in 1984 exposed people to methyl isocyanide gas, with health effects still felt today. Air pollution occurs when solid or gaseous contaminants are present in amounts that harm human health and the environment, and can be caused by both natural events like volcanoes and forest fires, as well
Types of plagiarism

The document defines and provides examples of various types of plagiarism, including identity theft, copycat plagiarism, cherry picking, mitosis, recycling, remixing, ghost citation, half n half, warping, mosaic plagiarism, reflections, miscues, half hearted plagiarism, 404 errors, incremental plagiarism, incidental plagiarism, self-plagiarism, incorrect citations, complete plagiarism, direct plagiarism, paraphrased plagiarism, mosaic plagiarism, and accidental plagiarism. Examples are given to illustrate direct, paraphrased, mosaic, self, and accidental plagiarism.
Plagiarism

The document discusses plagiarism, defining it as stealing and passing off others' ideas or words as one's own without attribution. It notes that plagiarism is unethical and can take various forms, such as substantial plagiarism where words are replaced with synonyms, or complete plagiarism where an entire work is presented as one's own. The document also discusses different types of plagiarism like accidental, self, and mosaic plagiarism. It provides strategies to avoid plagiarism like properly citing sources, paraphrasing while maintaining meaning, and acknowledging all contributions. Software tools to detect plagiarism are also outlined.
Designing the methodology

The document outlines the key steps in designing a research methodology, including formulating the research problem, conducting an extensive literature review, developing testable hypotheses, preparing the research design and sample, collecting and analyzing data, testing hypotheses, and preparing a final report. The goal is to systematically answer the research question by developing an experimental process to find the best solution under different conditions.
Experimental design techniques

Experimental design techniques involve controlling variables to measure their effects. There are three main types of designs: pre-experimental (no control group), quasi-experimental (no random assignment), and true experimental (control group, random assignment, manipulation). True experiments allow cause-and-effect conclusions through statistical analysis and include variations like post-test only, pre-test post-test, and Solomon four-group designs. Factorial designs test multiple hypotheses simultaneously by manipulating multiple independent variables. Randomized block and cross-over designs help control for differences between subjects. The goal is to draw valid conclusions about variable relationships through controlled experimentation.
All non parametric test

The Friedman test is a non-parametric statistical test used to detect differences in treatments across multiple test attempts. It compares three or more related/matched samples, such as the same measure repeated over time. The test ranks the data and calculates a statistic to determine if the null hypothesis that there are no differences between treatments can be rejected. For the given problem, the Friedman test was performed and calculated a chi-square value of 2.33, which is less than the critical value of 5.991 for 2 degrees of freedom. Therefore, the null hypothesis that there is no difference between the three weeks cannot be rejected at the 0.05 significance level.
Report writing

This document provides guidance on how to structure a research report. It should include the following main sections: introduction, methodology, results, conclusion, and appendices. The introduction states the purpose and rationale of the research. The methodology section describes the research design and methods. The results section reports the findings without bias. The conclusion summarizes key points and recommendations provide actionable steps. Appendices contain additional supporting materials like sources and references. Visual elements like charts and bullet points should also be used to enhance readability. The structure, language, and organization of each section is important to clearly convey the outcomes and significance of the research.
Observational study design

This document discusses different types of observational study designs used in epidemiology. It describes cohort studies, both prospective and retrospective, as well as case-control studies. Prospective cohort studies follow groups of subjects over time to observe outcomes based on exposures. Retrospective cohort studies use historical data to compare groups. Case-control studies compare cases with a disease to controls without the disease to identify risk factors. Cohort studies can precisely measure exposure-outcome relationships but are time-consuming and expensive. Observational studies are prone to biases like confounding, selection, information, and measurement bias.
Graphs17052021

This document discusses different types of statistical graphs used to visually represent numerical data. It describes graphs such as bar diagrams, pie charts, pictograms, maps, histograms, frequency polygons, frequency curves, line charts, and scatter diagrams. Bar diagrams can be simple, multiple, or proportional. Pie charts represent discrete data groups as sectors of a circle proportional to their frequencies. Pictograms and maps are used to show frequencies or distributions geographically. Histograms, polygons, and curves represent frequency distributions, while line charts show trends over time and scatter diagrams show correlations between two variables.
Measure of dispersion 10321

This document discusses measures of dispersion, which describe how widely or narrowly values are spread around the mean or median. It defines dispersion and explains that measures can be either absolute, measuring the amount of variation, or relative, measuring the degree of variation. Common measures discussed include range, standard deviation, and standard error. Standard deviation is presented as the most important absolute measure, calculated as the square root of the mean of squared deviations from the mean. Standard error measures variation between sample means. Examples are provided to demonstrate calculating different dispersion measures.
More from gopinathannsriramachandraeduin (20)
Recently uploaded
How to Setup Default Value for a Field in Odoo 17

In Odoo, we can set a default value for a field during the creation of a record for a model. We have many methods in odoo for setting a default value to the field.
Philippine Edukasyong Pantahanan at Pangkabuhayan (EPP) Curriculum

(𝐓𝐋𝐄 𝟏𝟎𝟎) (𝐋𝐞𝐬𝐬𝐨𝐧 𝟏)-𝐏𝐫𝐞𝐥𝐢𝐦𝐬
𝐃𝐢𝐬𝐜𝐮𝐬𝐬 𝐭𝐡𝐞 𝐄𝐏𝐏 𝐂𝐮𝐫𝐫𝐢𝐜𝐮𝐥𝐮𝐦 𝐢𝐧 𝐭𝐡𝐞 𝐏𝐡𝐢𝐥𝐢𝐩𝐩𝐢𝐧𝐞𝐬:
- Understand the goals and objectives of the Edukasyong Pantahanan at Pangkabuhayan (EPP) curriculum, recognizing its importance in fostering practical life skills and values among students. Students will also be able to identify the key components and subjects covered, such as agriculture, home economics, industrial arts, and information and communication technology.
𝐄𝐱𝐩𝐥𝐚𝐢𝐧 𝐭𝐡𝐞 𝐍𝐚𝐭𝐮𝐫𝐞 𝐚𝐧𝐝 𝐒𝐜𝐨𝐩𝐞 𝐨𝐟 𝐚𝐧 𝐄𝐧𝐭𝐫𝐞𝐩𝐫𝐞𝐧𝐞𝐮𝐫:
-Define entrepreneurship, distinguishing it from general business activities by emphasizing its focus on innovation, risk-taking, and value creation. Students will describe the characteristics and traits of successful entrepreneurs, including their roles and responsibilities, and discuss the broader economic and social impacts of entrepreneurial activities on both local and global scales.
A Free 200-Page eBook ~ Brain and Mind Exercise.pptx

(A Free eBook comprising 3 Sets of Presentation of a selection of Puzzles, Brain Teasers and Thinking Problems to exercise both the mind and the Right and Left Brain. To help keep the mind and brain fit and healthy. Good for both the young and old alike.
Answers are given for all the puzzles and problems.)
With Metta,
Bro. Oh Teik Bin 🙏🤓🤔🥰
Educational Technology in the Health Sciences

Plenary presentation at the NTTC Inter-university Workshop, 18 June 2024, Manila Prince Hotel.
Level 3 NCEA - NZ: A Nation In the Making 1872 - 1900 SML.ppt

The History of NZ 1870-1900.
Making of a Nation.
From the NZ Wars to Liberals,
Richard Seddon, George Grey,
Social Laboratory, New Zealand,
Confiscations, Kotahitanga, Kingitanga, Parliament, Suffrage, Repudiation, Economic Change, Agriculture, Gold Mining, Timber, Flax, Sheep, Dairying,
How to Manage Reception Report in Odoo 17

A business may deal with both sales and purchases occasionally. They buy things from vendors and then sell them to their customers. Such dealings can be confusing at times. Because multiple clients may inquire about the same product at the same time, after purchasing those products, customers must be assigned to them. Odoo has a tool called Reception Report that can be used to complete this assignment. By enabling this, a reception report comes automatically after confirming a receipt, from which we can assign products to orders.
BÀI TẬP BỔ TRỢ TIẾNG ANH LỚP 8 - CẢ NĂM - FRIENDS PLUS - NĂM HỌC 2023-2024 (B...

BÀI TẬP BỔ TRỢ TIẾNG ANH LỚP 8 - CẢ NĂM - FRIENDS PLUS - NĂM HỌC 2023-2024 (B...Nguyen Thanh Tu Collection
https://app.box.com/s/nrwz52lilmrw6m5kqeqn83q6vbdp8yzpHow to Fix [Errno 98] address already in use![How to Fix [Errno 98] address already in use](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![How to Fix [Errno 98] address already in use](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
This slide will represent the cause of the error “[Errno 98] address already in use” and the troubleshooting steps to resolve this error in Odoo.
220711130083 SUBHASHREE RAKSHIT Internet resources for social science

Internet resources for social science
Bossa N’ Roll Records by Ismael Vazquez.

Bossa N Roll Records presentation by Izzy Vazquez for Music Retail and Distribution class at Full Sail University
CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...

CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...Nguyen Thanh Tu Collection
https://app.box.com/s/qspvswamcohjtbvbbhjad04lg65waylfRecently uploaded (20)
REASIGNACION 2024 UGEL CHUPACA 2024 UGEL CHUPACA.pdf

REASIGNACION 2024 UGEL CHUPACA 2024 UGEL CHUPACA.pdf
220711130088 Sumi Basak Virtual University EPC 3.pptx

220711130088 Sumi Basak Virtual University EPC 3.pptx
Philippine Edukasyong Pantahanan at Pangkabuhayan (EPP) Curriculum

Philippine Edukasyong Pantahanan at Pangkabuhayan (EPP) Curriculum
A Free 200-Page eBook ~ Brain and Mind Exercise.pptx

A Free 200-Page eBook ~ Brain and Mind Exercise.pptx
Level 3 NCEA - NZ: A Nation In the Making 1872 - 1900 SML.ppt

Level 3 NCEA - NZ: A Nation In the Making 1872 - 1900 SML.ppt
BÀI TẬP BỔ TRỢ TIẾNG ANH LỚP 8 - CẢ NĂM - FRIENDS PLUS - NĂM HỌC 2023-2024 (B...

BÀI TẬP BỔ TRỢ TIẾNG ANH LỚP 8 - CẢ NĂM - FRIENDS PLUS - NĂM HỌC 2023-2024 (B...
220711130083 SUBHASHREE RAKSHIT Internet resources for social science

220711130083 SUBHASHREE RAKSHIT Internet resources for social science
CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...

CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...
Mass spectrometry
- 2. Weight The most common definition of weight found in introductory physics textbooks defines weight as the force exerted on a body by gravity. An object's weight depends on its mass and the strength of the gravitational pull. The force with which an object near the Earth or another celestial body is attracted toward the center of the body by gravity.
- 3. Mass • Mass is both a property of a physical body and a measure of its resistance to acceleration (a change in its state of motion) when a net force is applied. An object's mass also determines the strength of its gravitational attraction to other bodies. The basic SI unit of mass is the kilogram (kg).
- 8. • Mass spectrometry is a microanalytical technique that can be used selectively to detect and determine the amount of a given analyte. • Mass spectrometry is also used to determine the elemental composition and some aspects of the molecular structure of an analyte. • The tools of mass spectrometry are mass spectrometers, and the data are mass spectra.
- 9. • The experimental measurement of the mass of gas-phase ions produced from molecules of an analyte. • Mass spectrometry concerns itself with the mass of the isotopes of the elements, not the atomic mass of the elements.
- 10. Atomic number
- 11. Atomic number The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons.
- 14. Atomic mass • The atomic mass of an element is the weighted average of the naturally occurring stable isotopes that comprise the element.
- 15. Ionization • Ionization or ionisation, is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes.
- 19. • Mass spectrometry does not directly determine mass; it determines the mass-to- charge ratio (m/z) of ions. • It is a fundamental requirement of mass spectrometry that the ions be in the gas phase before they can be separated according to their individual m/z values and detected.
- 20. Before 1970- ionization techniques • Analytes having significant vapor pressure were amenable to mass spectrometry because gas-phase ions could only be produced from gas-phase molecules by the techniques of electron ionization (EI) or chemical ionization (CI). • Nonvolatile and thermally labile molecules were not amenable.
- 21. After 1970- Desorption techniques • After 1970, the capabilities of mass spectrometry were expanded by the development of desorption/ionization (D/I)techniques, the generic process of generating gas-phase ions directly from a sample in the condensed phase. • The first viable and widely accepted technique2 for D/I was fast atom bombardment (FAB), which required nanomoles of analyte to produce an interpretable mass spectrum.
- 22. In 1980 • During the 1980s, electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) eclipsed FAB, in part because they required only picomoles of analyte for analysis. • ESI and MALDI are suitable for analysis of femtomole quantities of thermally labile and nonvolatile analytes
- 23. The Concept of Mass Spectrometry • Ions are charged particles and, as such, their position in space can be manipulated with the use of electric and magnetic fields. • When only individual ions are present, they can be grouped according to their unique properties (mass and the number of charges) and moved from one point to another. • In order to have individual ions free from any other forms of matter, it is necessary to analyze them in a vacuum.
- 24. • Mass spectrometry takes advantage of ions in the gas phase at low pressures to separate and detect them according to their • mass-to-charge ratio (m/z) – the mass of the ion on the atomic scale divided by the number of charges that the ion possesses.
- 25. • Only ions are detected in mass spectrometry. • Any particles that are not ionic (molecules or radicals ) are removed from the mass spectrometer by the continuous pumping that maintains the vacuum. • Both molecules and radicals are particles that have no charge. • Molecules are characterized by an even number of electrons . • Radicals by an odd number of electrons.
- 26. • The mass component that makes up the dimensionless m/z unit is based on an atomic scale rather than the physical scale. • Mass physical scale is defined as one kilogram being the mass of one liter of water at a specific temperature and pressure. • The atomic mass scale is defined based on a fraction of a specific isotope of carbon; • i.e., 1 mass unit on an atomic scale is equal to 1/12 the mass of the most abundant naturally occurring stable isotope of carbon, 12C. This definition of mass. • Mass is represented by the symbol u, which is synonymous with dalton(Da).
- 27. • In the study of mass spectrometry, it is important to always keep in mind that the entity measured in the mass spectrometer is the mass-to-charge ratio of an ion, not the mass of the ion. • It is inappropriate to use a unit of mass when describing the mass-to-charge ratio of an ion. Ions have both mass and an m/z value.
- 28. M+ ions • The most common ionization process for gas- phase analysis, • EI, transfers energy to the neutral molecule (a species characterized as having an even number of electrons) in the vapor state, giving it sufficient energy to eject one of its own electrons, thereby leaving a residual positive charge on the now ionic species. This process produces a molecular ion with a positive charge and odd number of electrons, as represented by the M+
- 29. • This M + may have considerable excess energy that can be dissipated through fragmentation of certain chemical bonds. • Cleavage of various chemical bonds leads to the production of positive-charge fragment ions whose mass is equal to the sum of the atomic masses of the constituent atoms. • Not all of the molecular ions necessarily decompose into fragment ions. For compounds producing a relatively stable M +, such as those stabilized through resonance, like aromatic compounds, an intense molecular-ion peak will be recorded because the M+ tends to survive or resist fragmentation. • For compounds that do not produce stable molecular ions, like aliphatic alcohols, nearly all of them decompose into fragment ions. In these cases, the mass spectrum contains only a small peak representing the M+ Various combinations of the above-described processes are the basis of the chemical “fingerprint” in the form of a mass spectrum for a given compound.
- 30. Calutron mass spectrometers were used in the Manhattan Project for uranium enrichment.
- 31. Replica of J.J. Thomson's third mass spectrometer
- 32. Surface ionization source at the Argonne National Laboratory linear accelerator
- 34. ISOTOPES
- 35. • An element is specified by the number of protons in its nucleus. This equals the atomic number of the respective element, and thus determines its place within the periodic table of the elements. The atomic number is given as a subscript preceding the elemental symbol, e.g., 6C in case of carbon.
- 36. • Atoms with nuclei of the same atomic number differing in the number of neutrons are termed isotopes. • The mass number of an isotope is given as superscript preceding the elemental symbol, e.g., 12C.
- 37. Monoisotopic elements Elements do exist in only one naturally occurring stable isotope. • Fluorine (19F), • Sodium (23Na), • Phosphorus (31P) • Iodine (127I) • The monoisotopic elements are also referred to as A or X elements
- 38. Di-isotopic Elements • Several elements naturally exist in two isotopes. • The first group has been termed A+1 or X+1 elements, the latter ones have been termed A+2 or X+2 elements.
- 39. X+1 Elements • Hydrogen (1H, 2H = D), carbon (12C, 13C) and nitrogen (14N, 15N). • Deuterium (D) is of low abundance (0.0115 %) • And therefore, hydrogen is usually treated as monoisotopic or X element
- 40. X+2 Elements • Chlorine (35cl, 37cl) • Bromine (79br, 81br) are Commonly Known, • Copper (63cu, 65cu), • Gallium (69ga, 71ga), • Silver (107ag, 109ag), • Indium (113in, 115in) and • Antimony (121Sb, 123Sb)
- 41. X-1 element The elements lithium (6Li, 7Li), boron (10B, 11B) and vanadium (50V, 51V) come together with a lighter isotope of lower abundance than the heavier one and thus, they can be grouped together as X–1 elements.
- 42. Polyisotopic Elements The majority of elements belongs to the polyisotopic elements because they consist of three or more isotopes showing a wide variety of isotopic distributions.
- 43. The isotopic mass • It is the exact mass of an isotope. • It is very close to but not equal to the nominal mass of the isotope . • The only exception is the carbon isotope 12C which has an isotopic mass of 12.000000 u.
- 44. Monoisotopic Mass The exact mass of the most abundant isotope of an element is termed monoisotopic mass.