The document provides an overview of chemical oceanography, including:
1) Chemical oceanography is the study of interactions between elements and the world's oceans.
2) Seawater contains nearly every element on Earth and has a consistent salt concentration regardless of location.
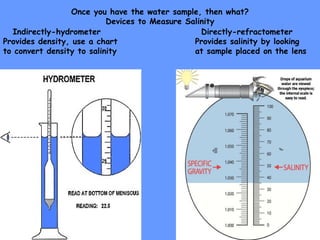
3) Tools like CTD probes, Niskin bottles, and refractometers are used to measure properties like salinity, density, and dissolved oxygen levels at different ocean depths.
4) Low dissolved oxygen (hypoxia) can create "dead zones" harmful to marine life.