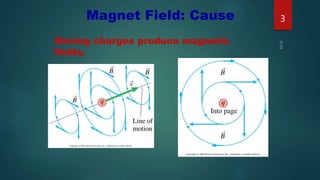
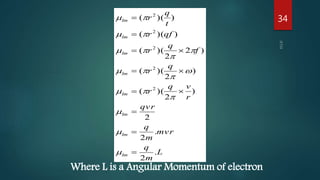
1) Magnetism arises due to the orbital and spin motion of electrons in materials. The orbital motion of electrons gives rise to orbital magnetic moments, while the spin of electrons and nuclei gives rise to spin magnetic moments.
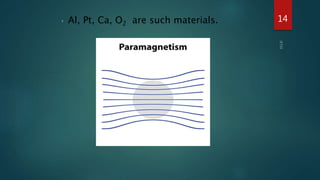
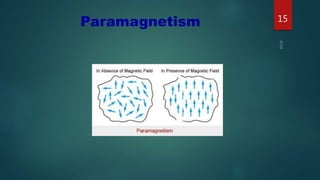
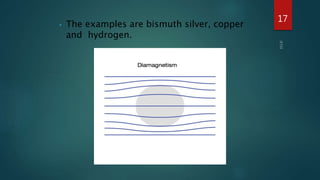
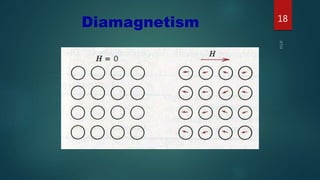
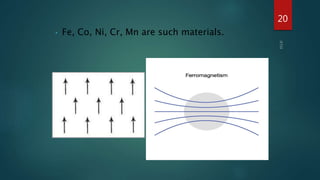
2) Magnetic materials can be classified as diamagnetic, paramagnetic, ferromagnetic, ferrimagnetic, or antiferromagnetic depending on their magnetic susceptibility and behavior in an applied magnetic field. Ferromagnetic materials like iron have the largest susceptibility.
3) The magnetic induction B in a material is proportional to the applied magnetic field strength H, with the constant of proportionality being the permeability μ of the material. The ratio of μ of a material to the permeability of free space is known as