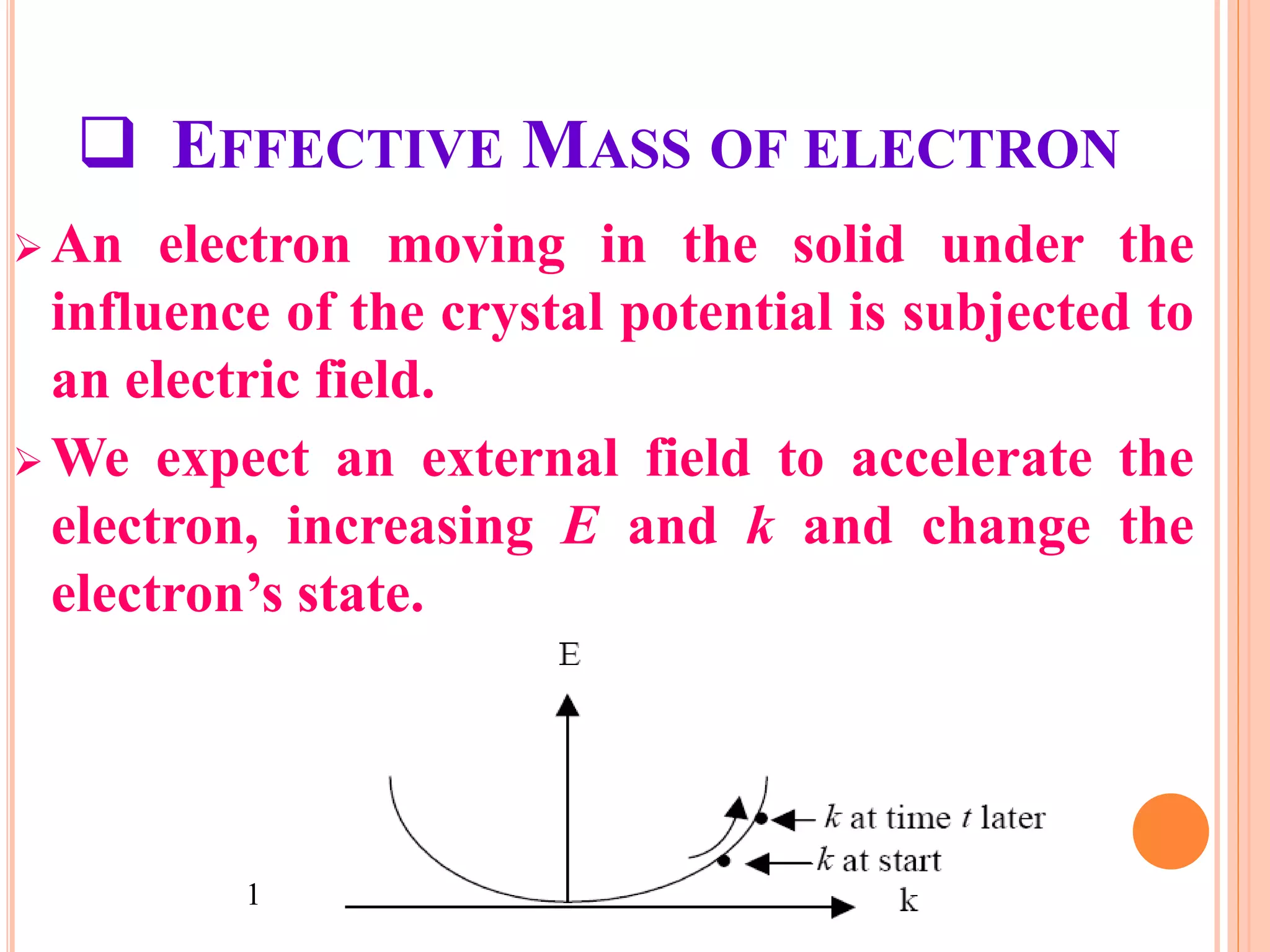
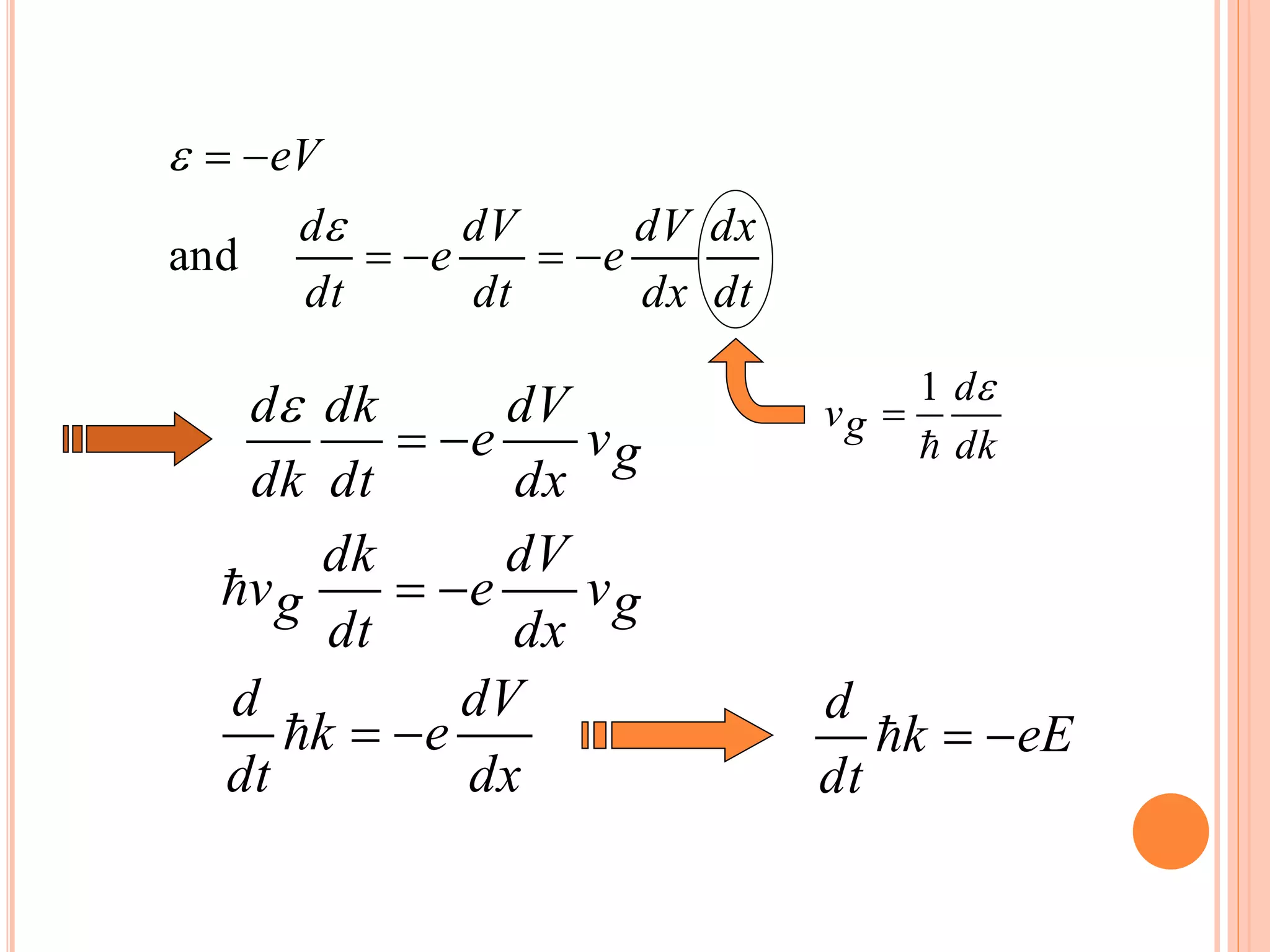
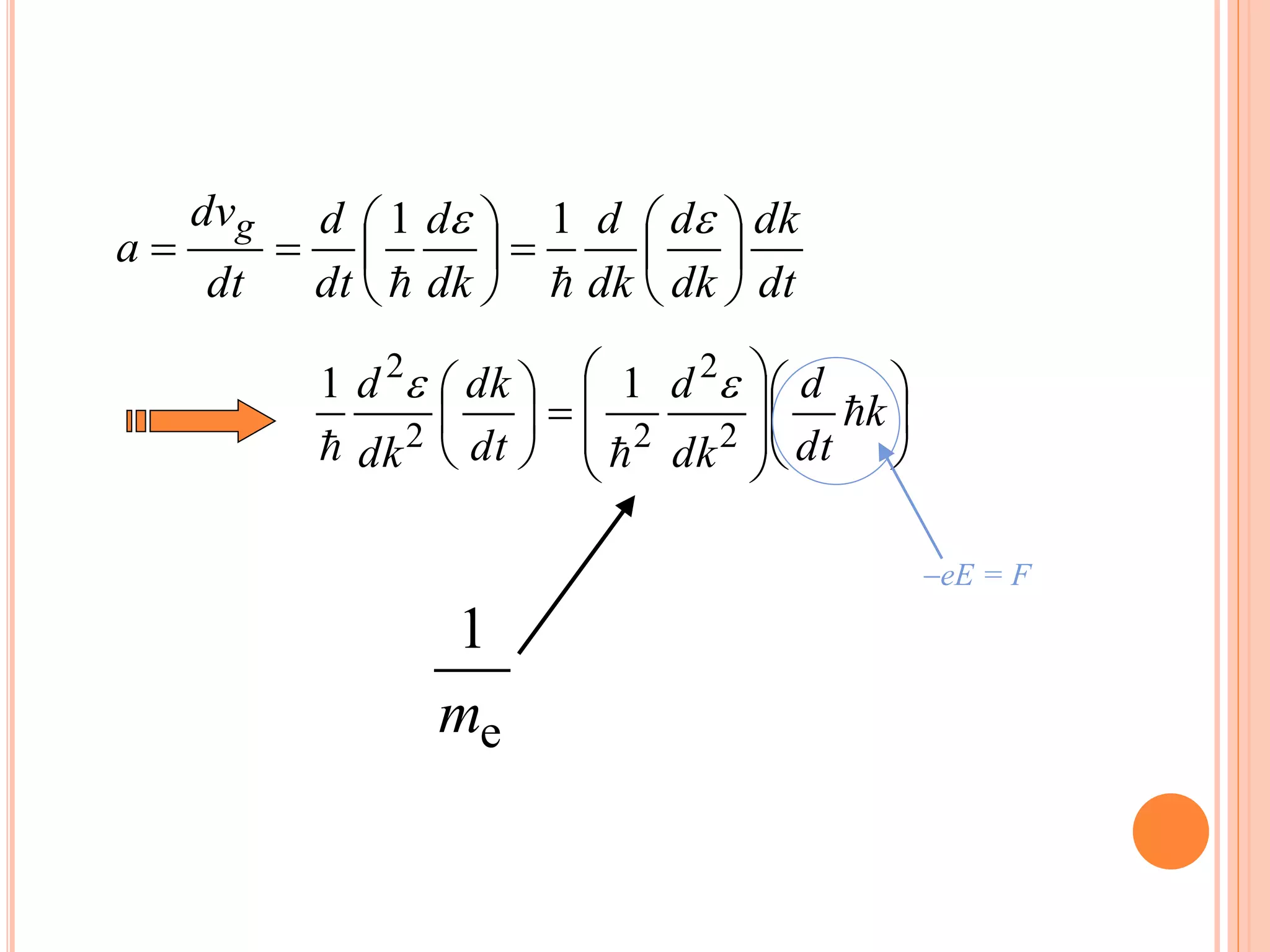
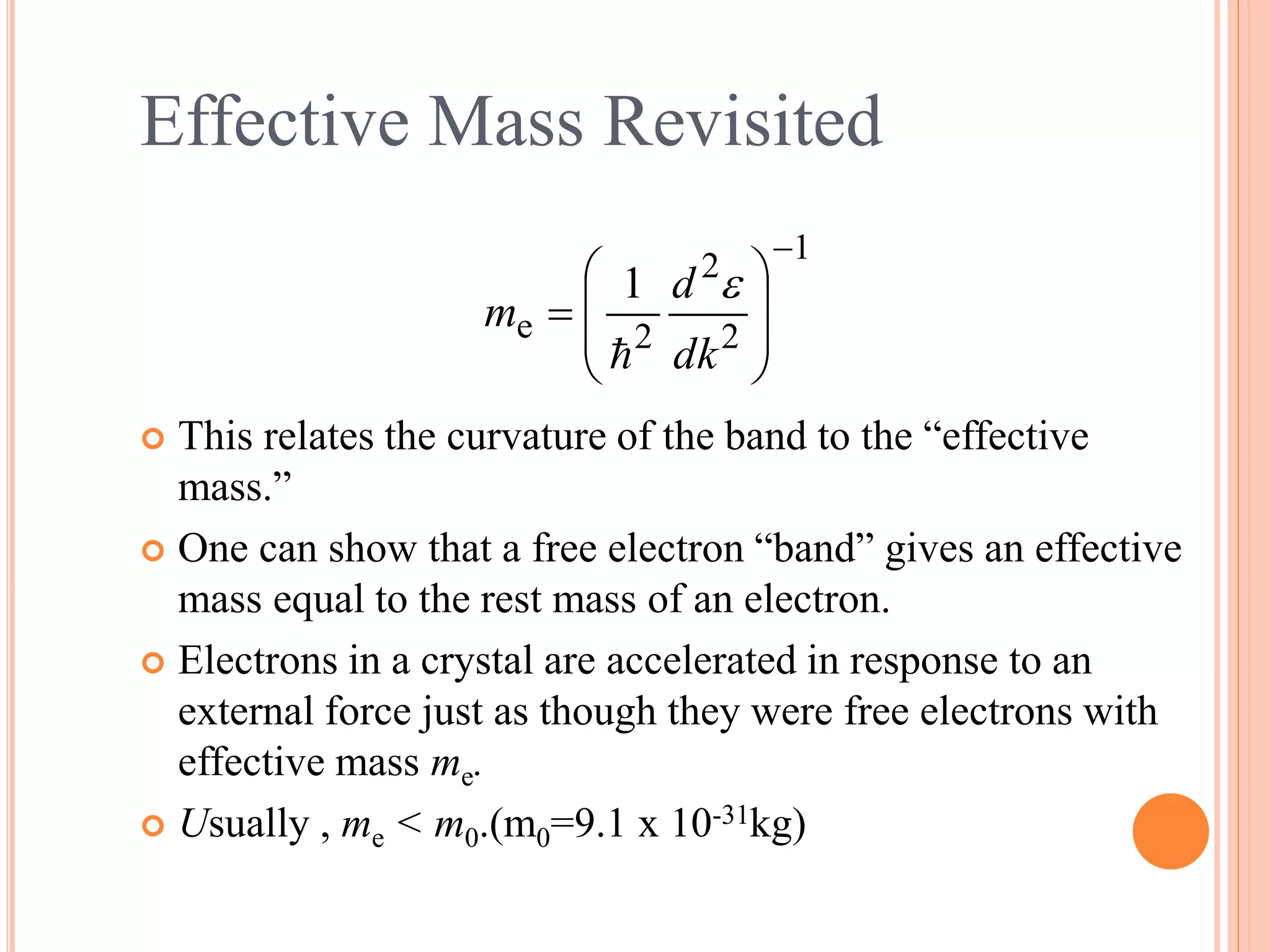
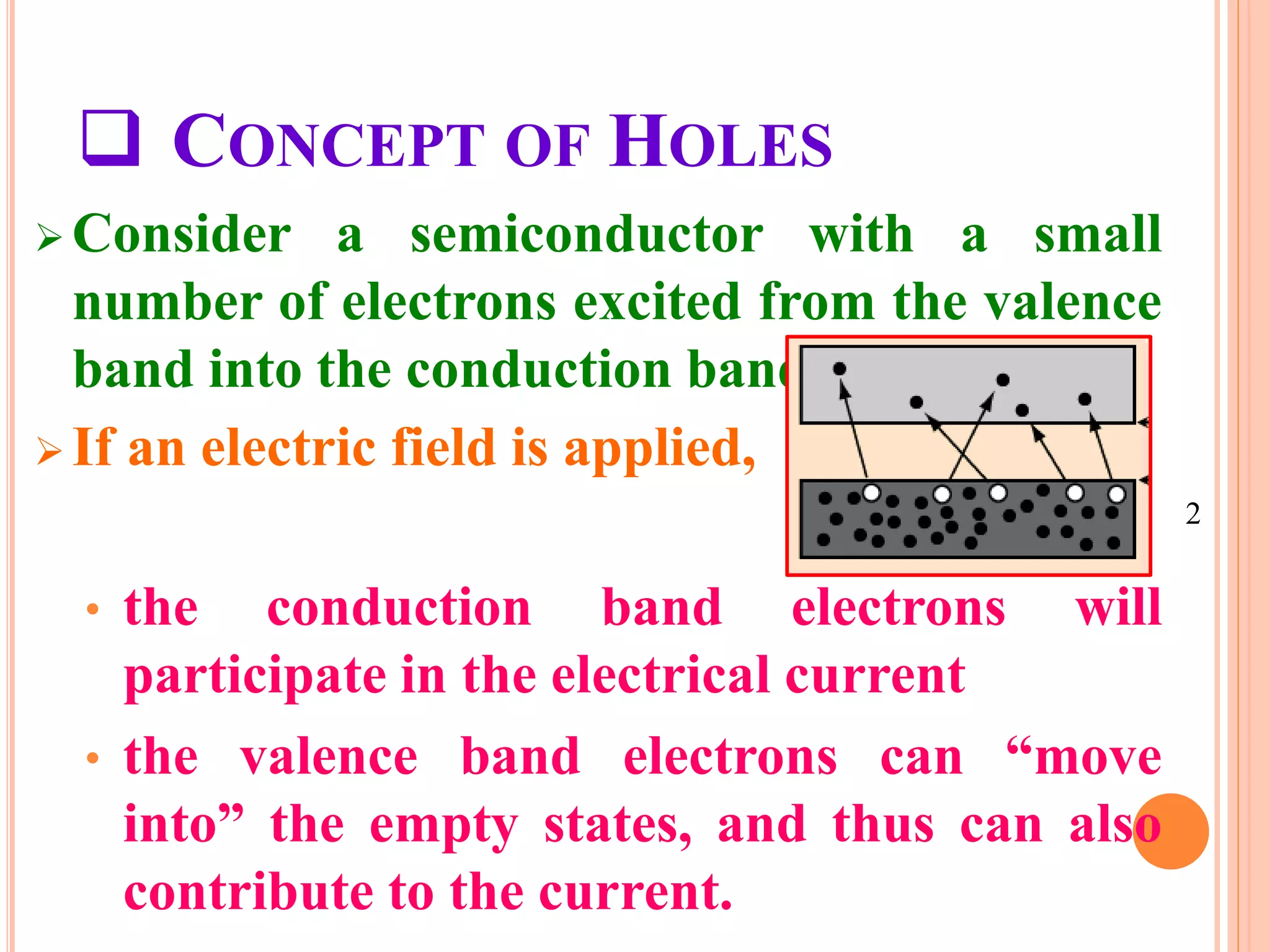
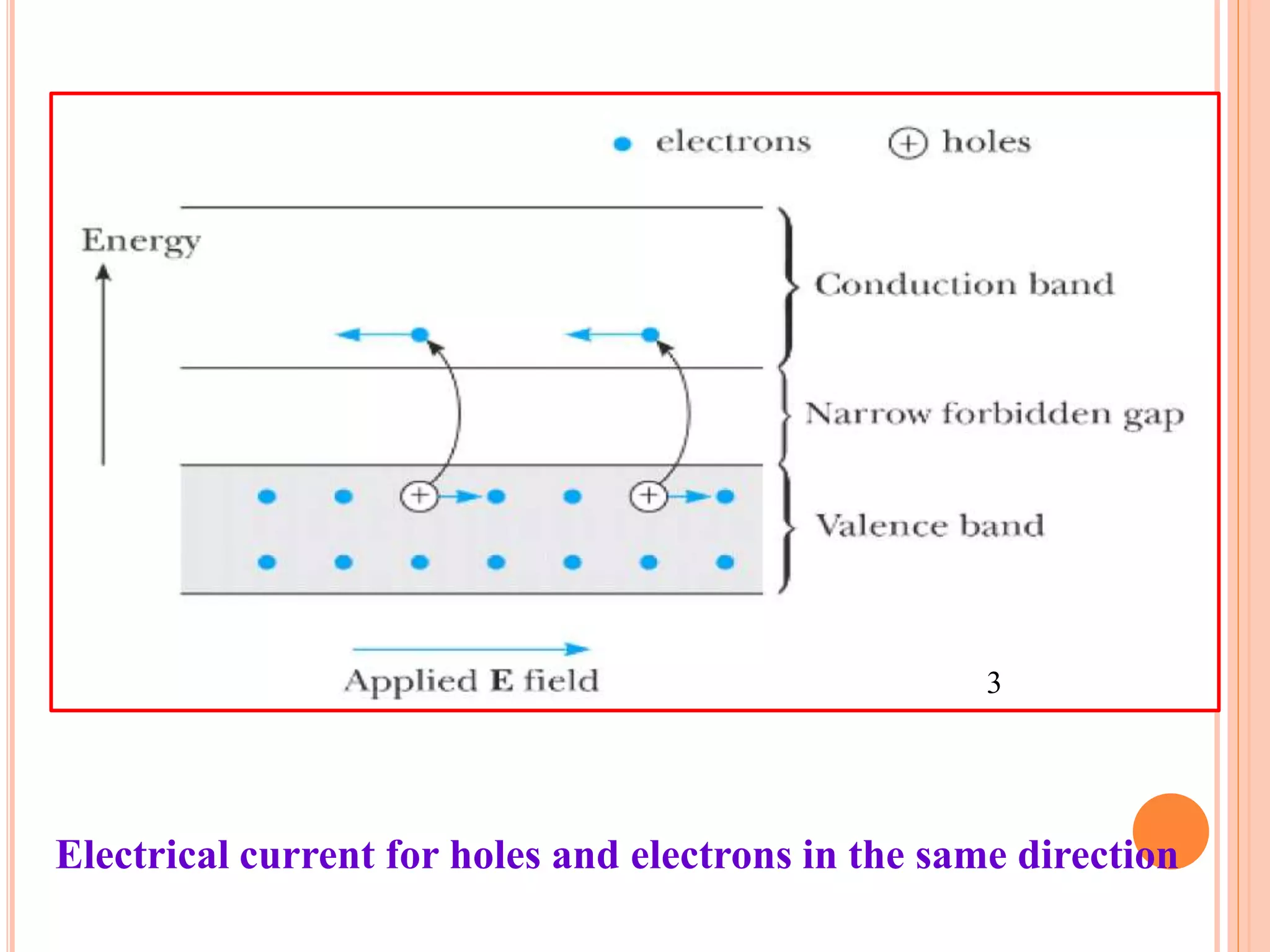
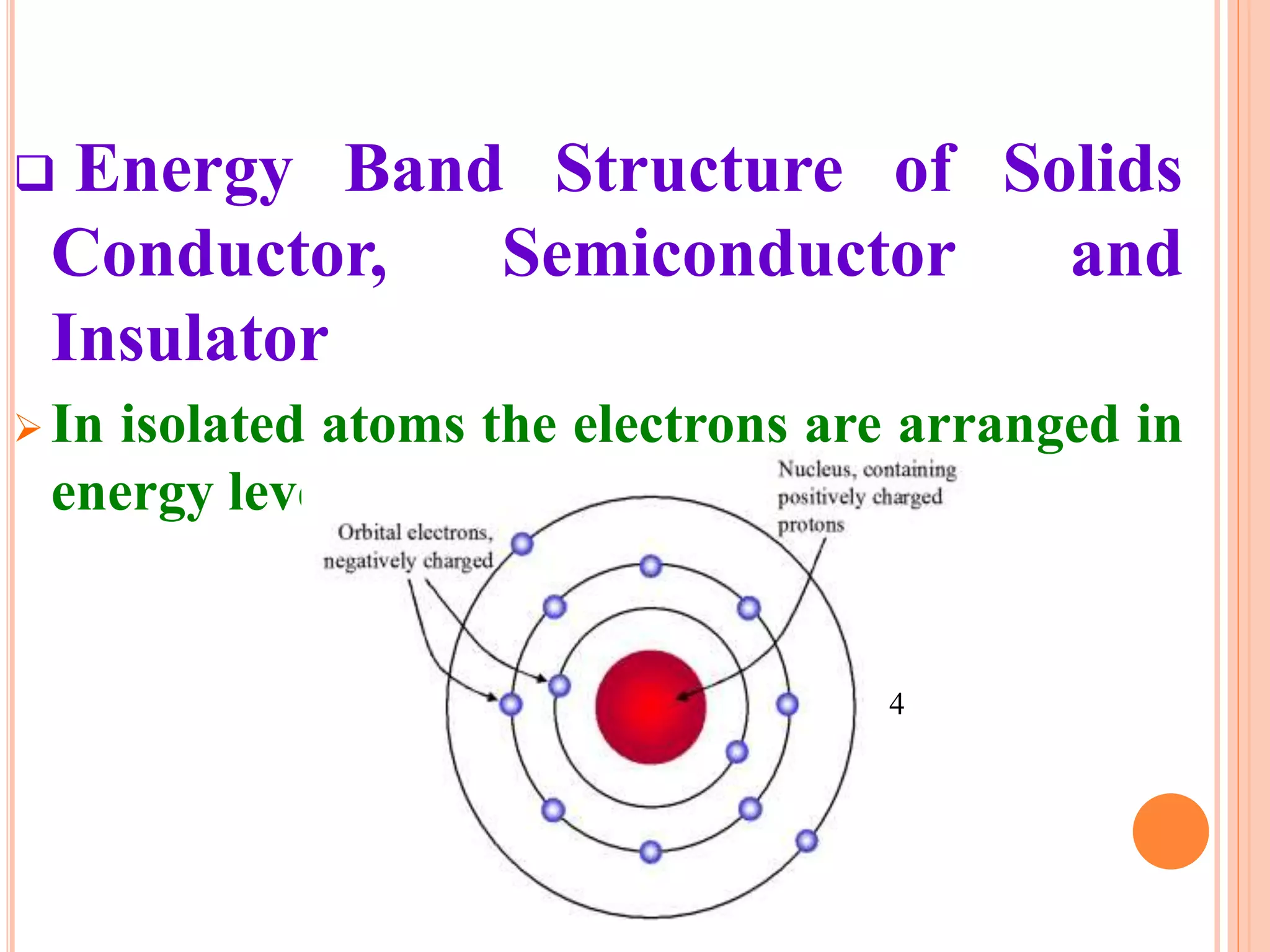
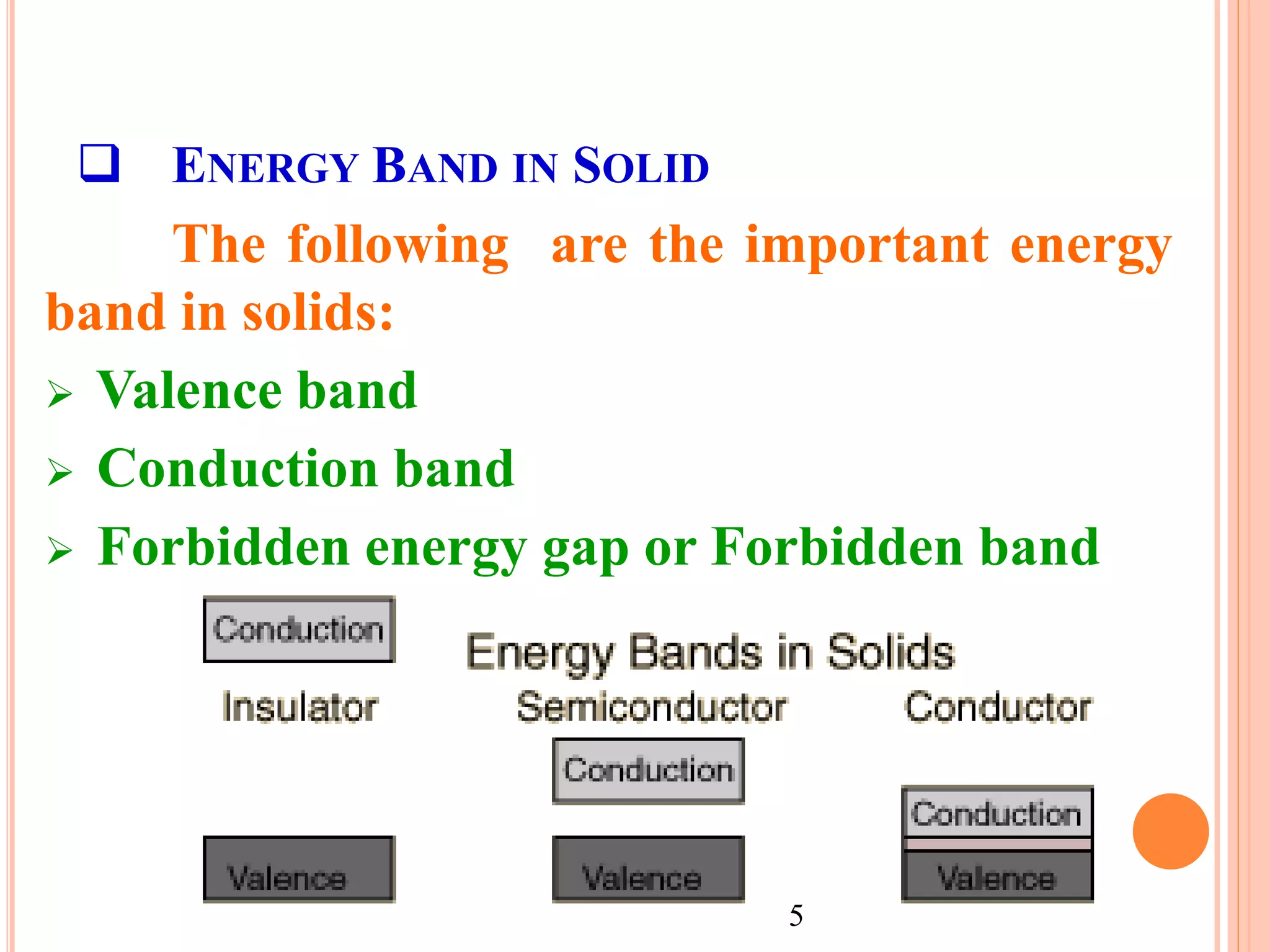
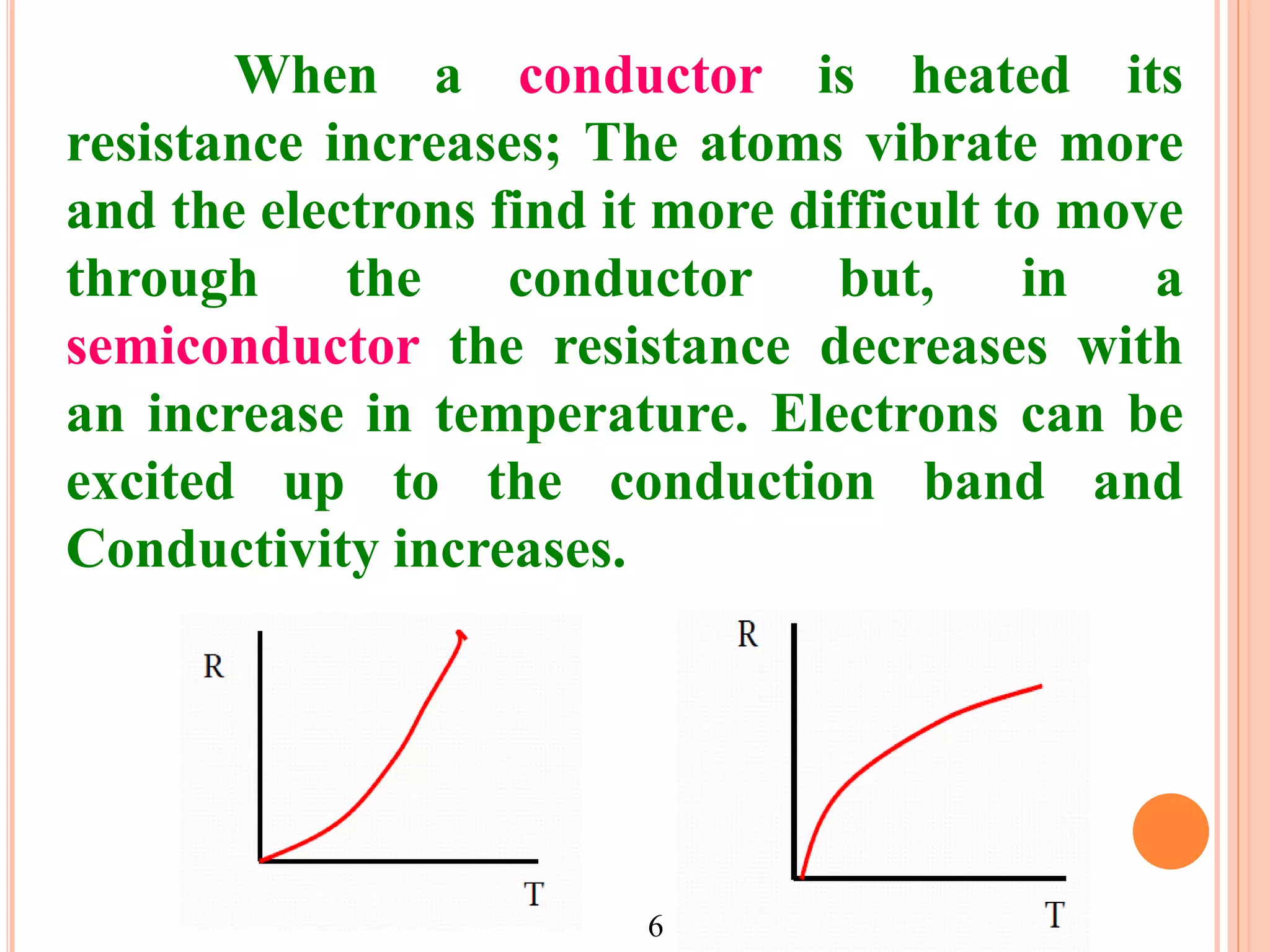
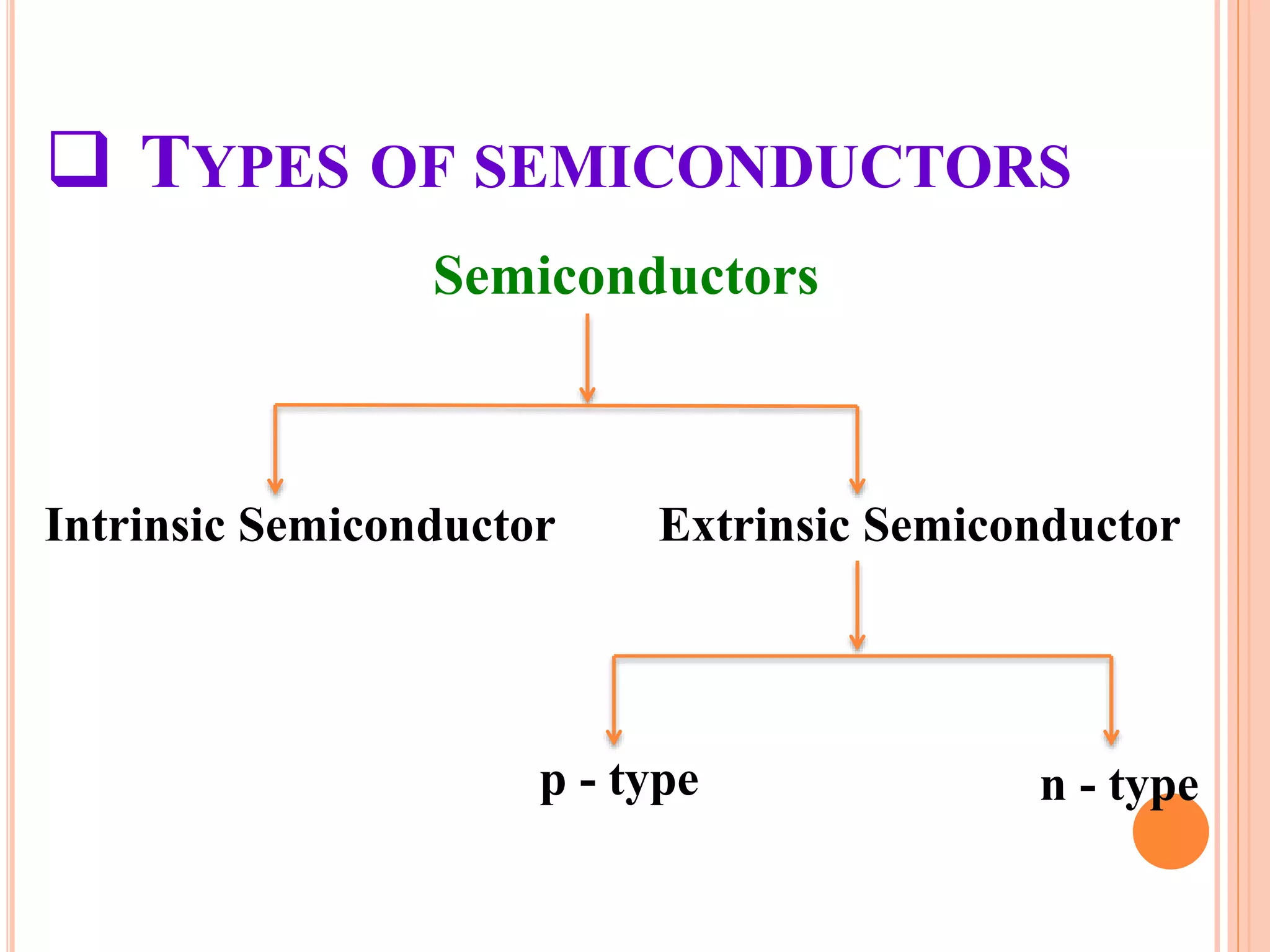
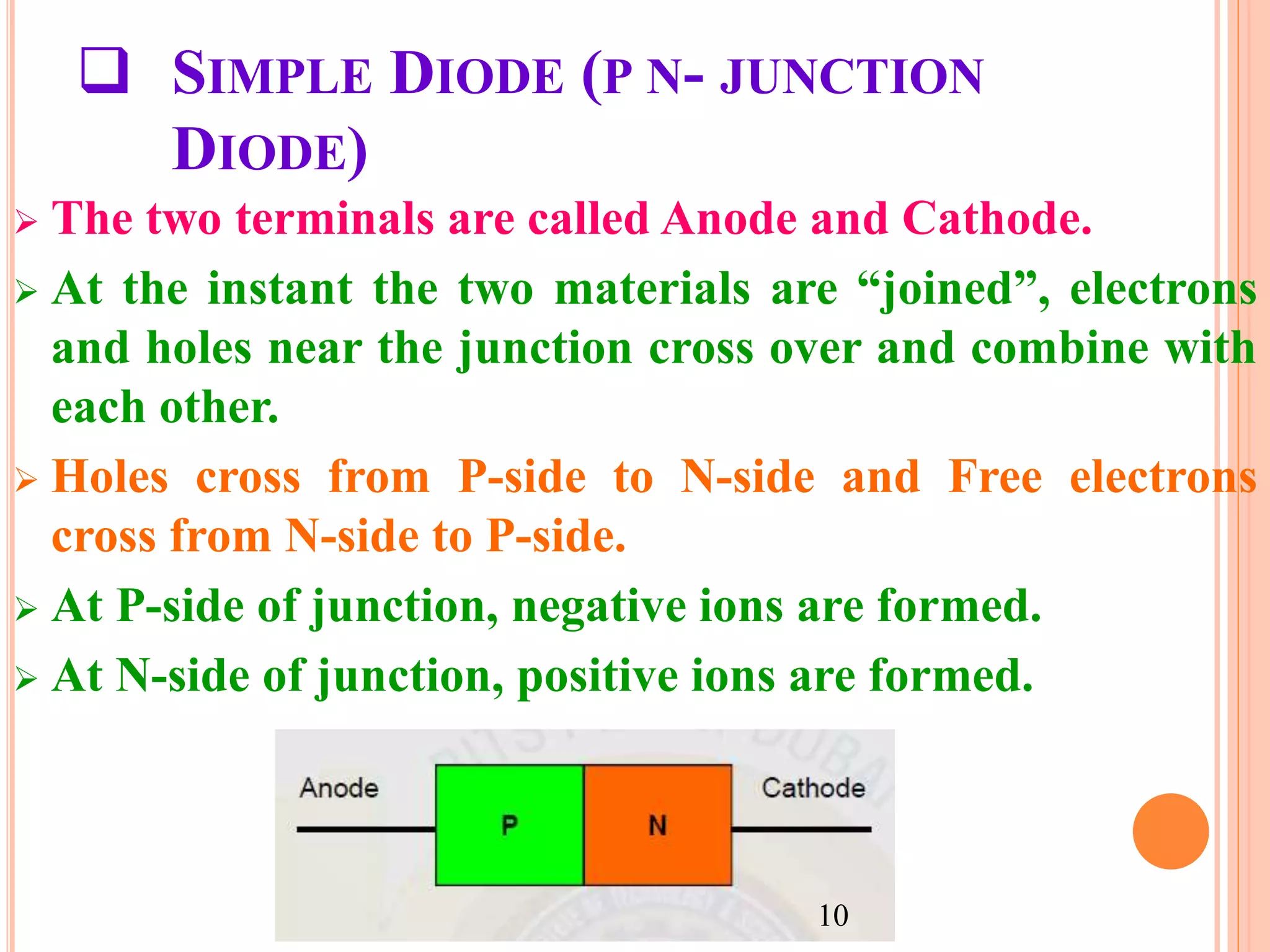
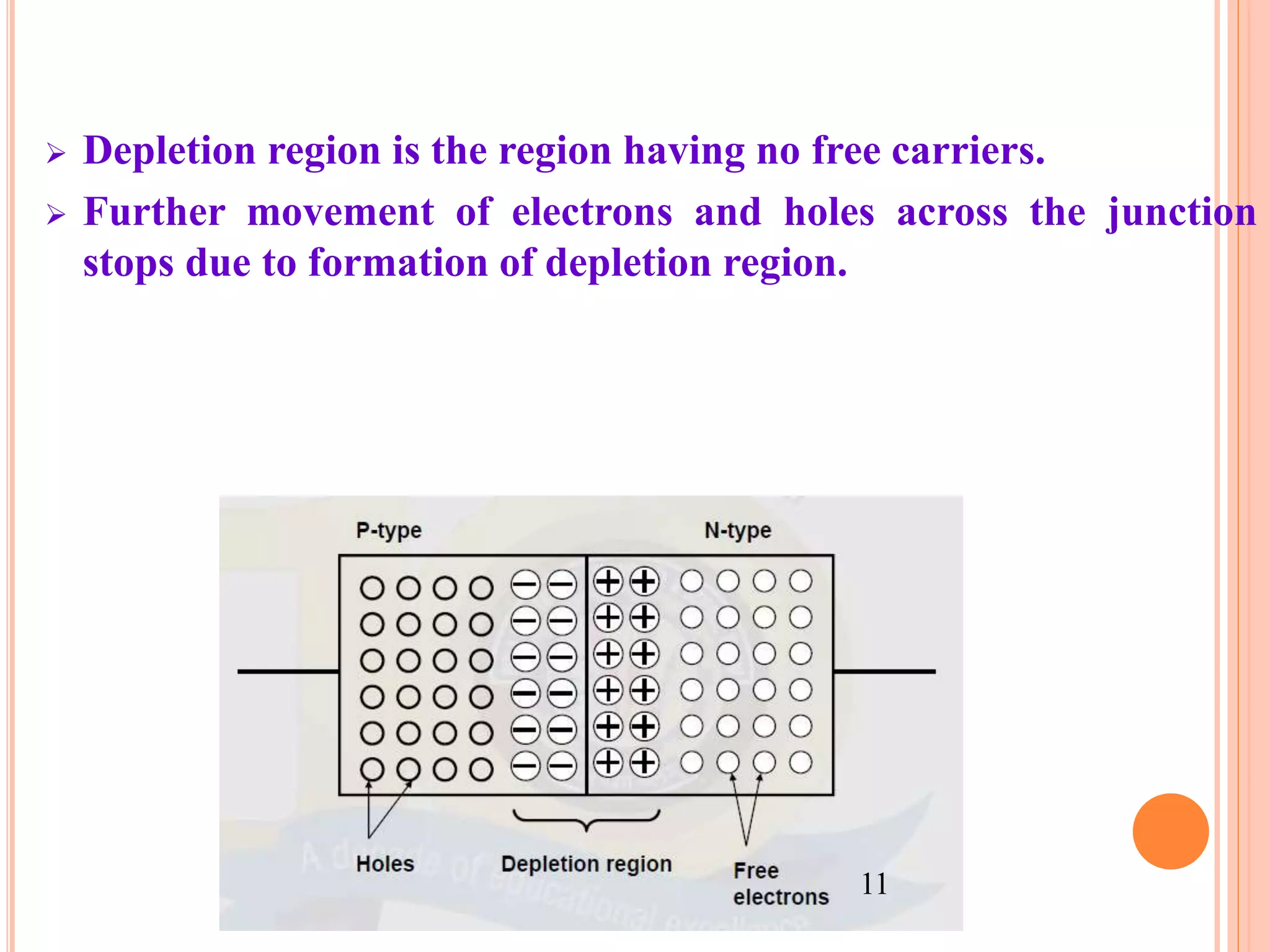
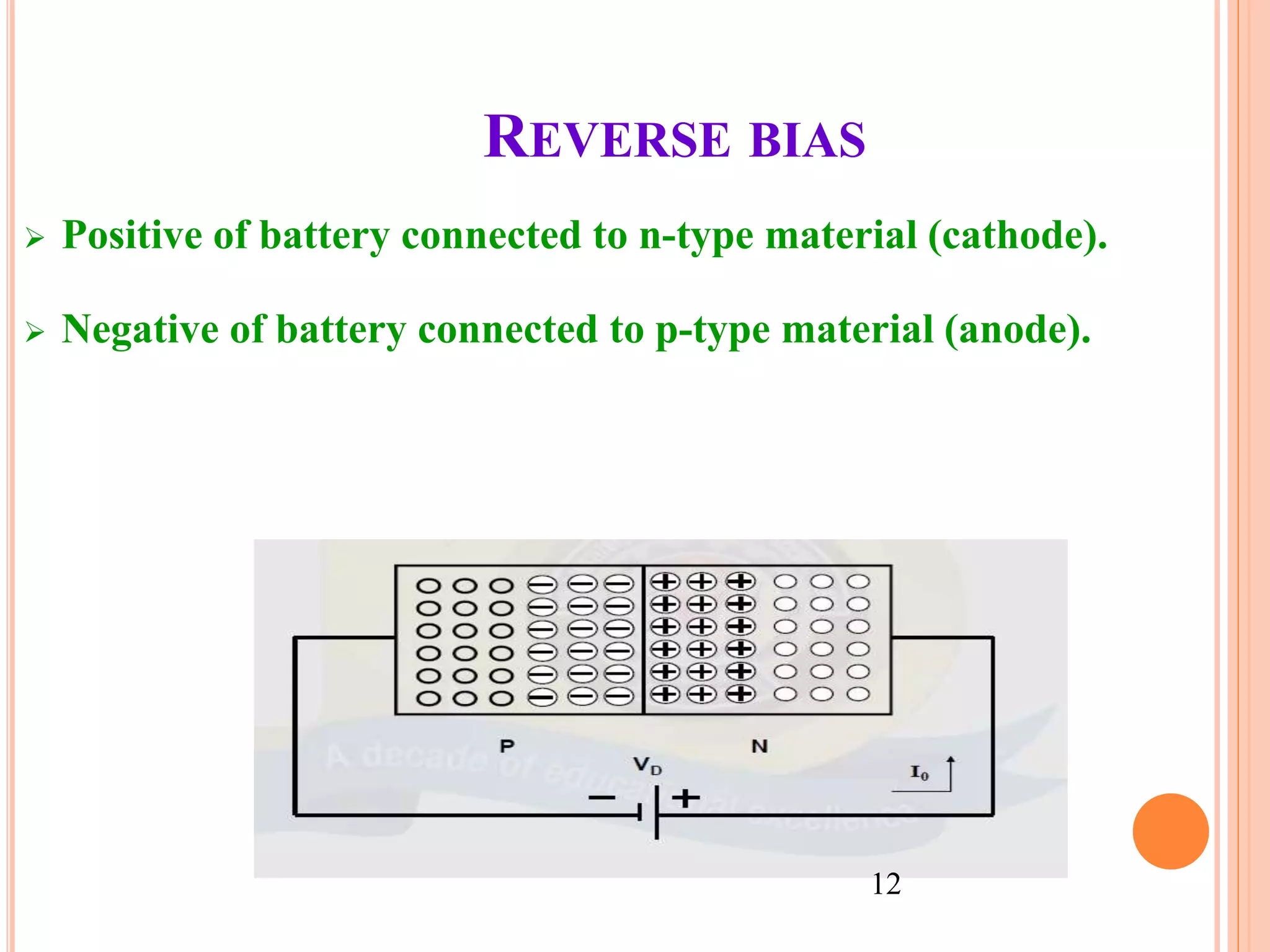
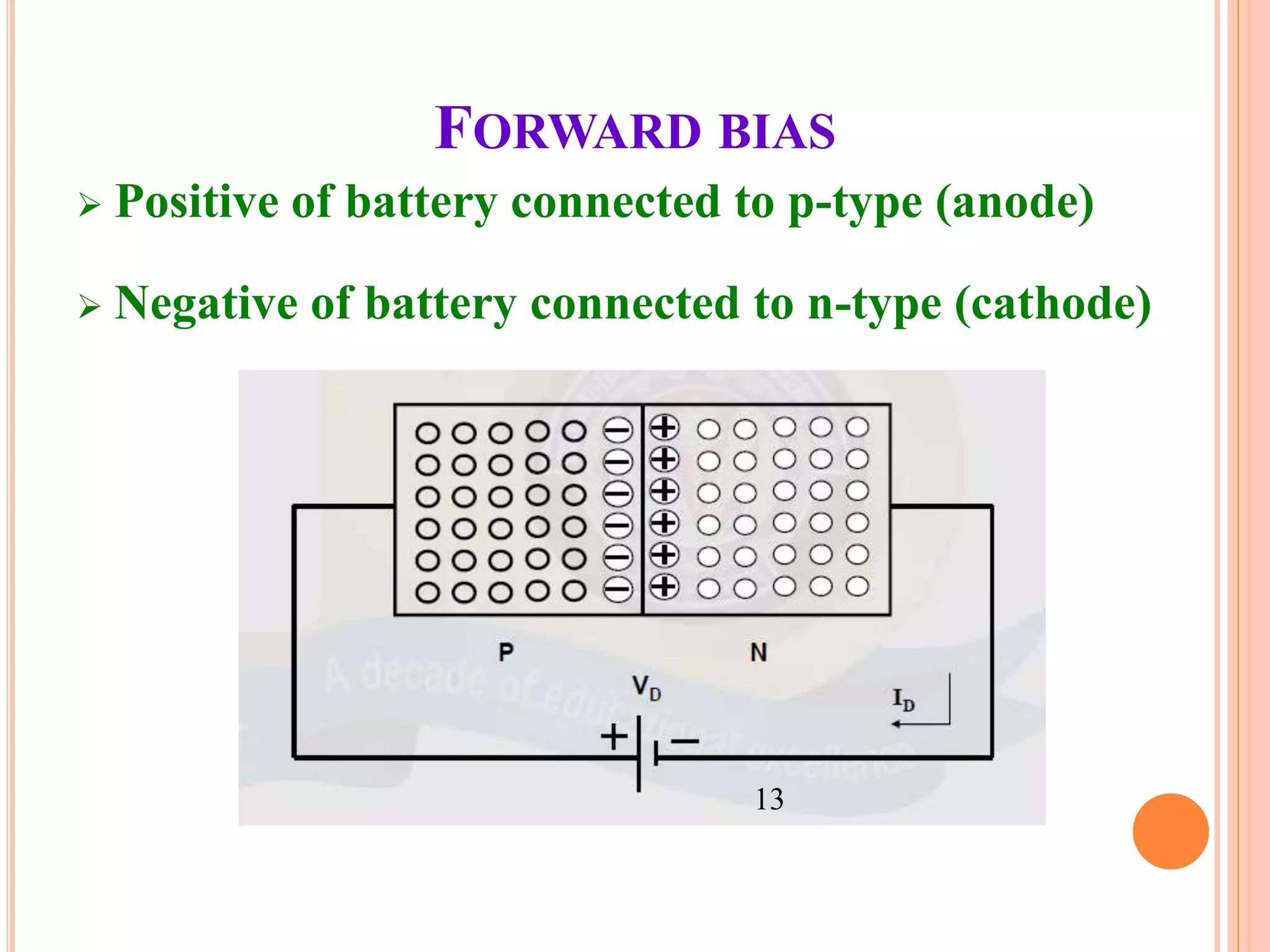
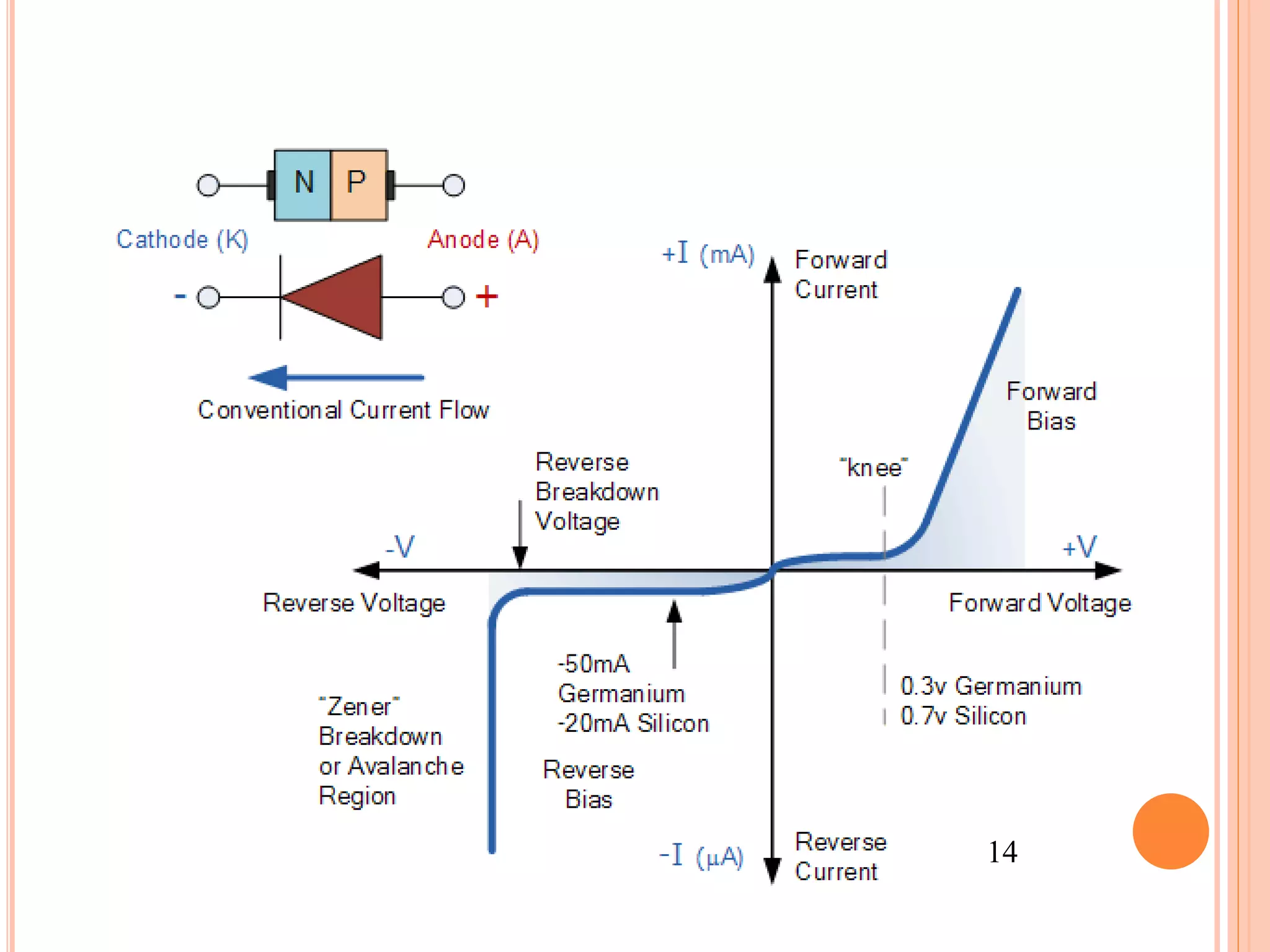
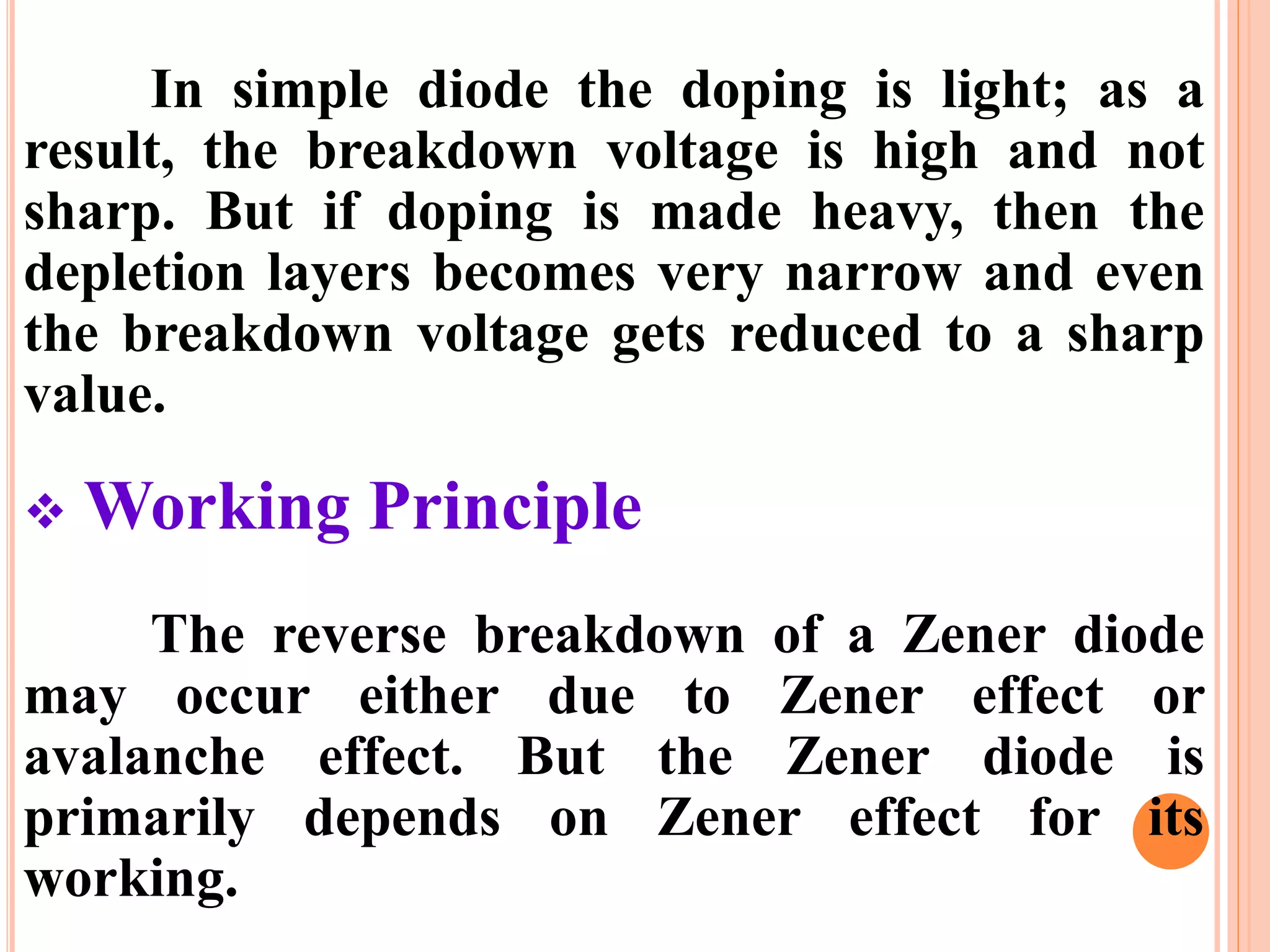
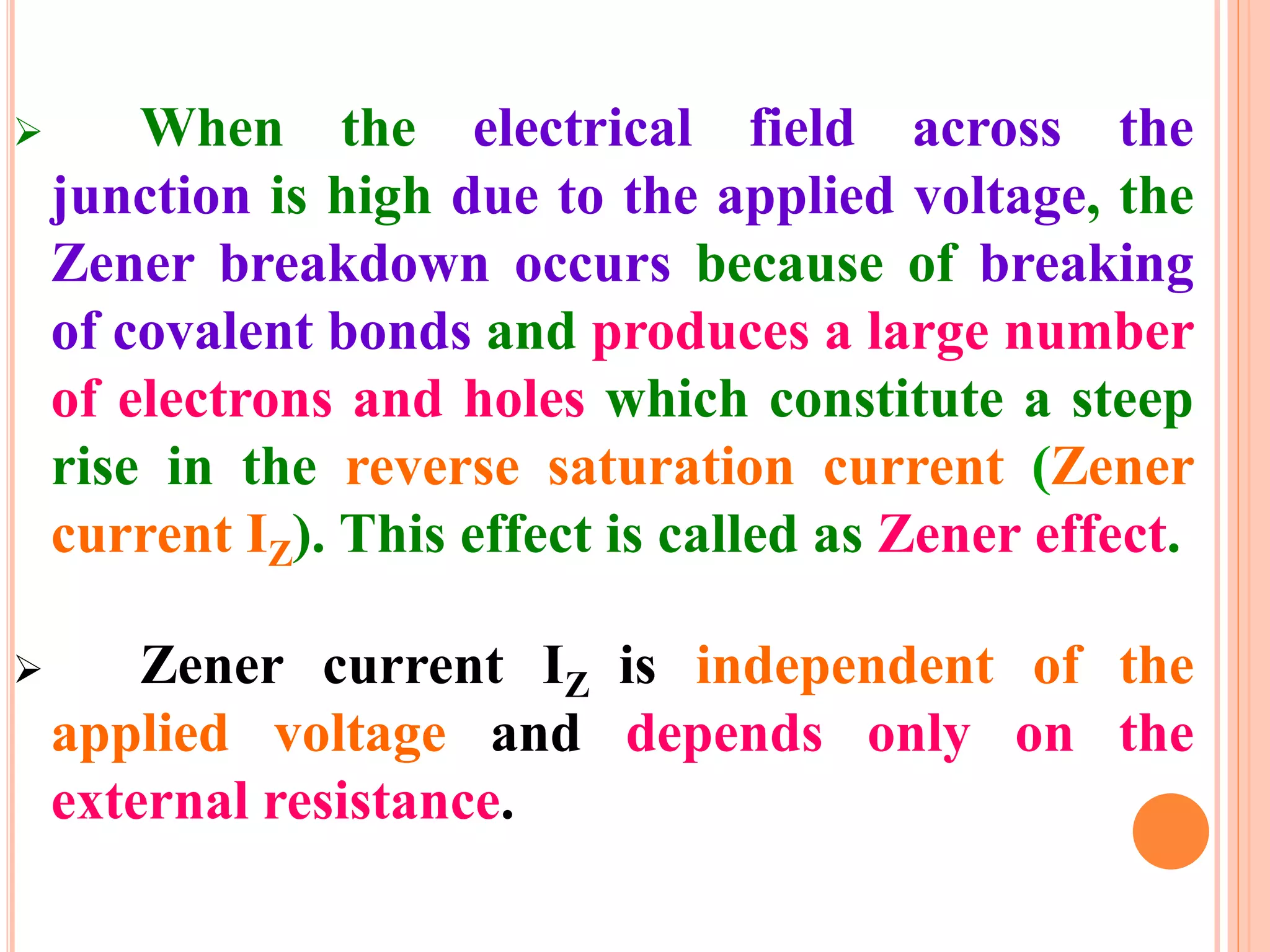
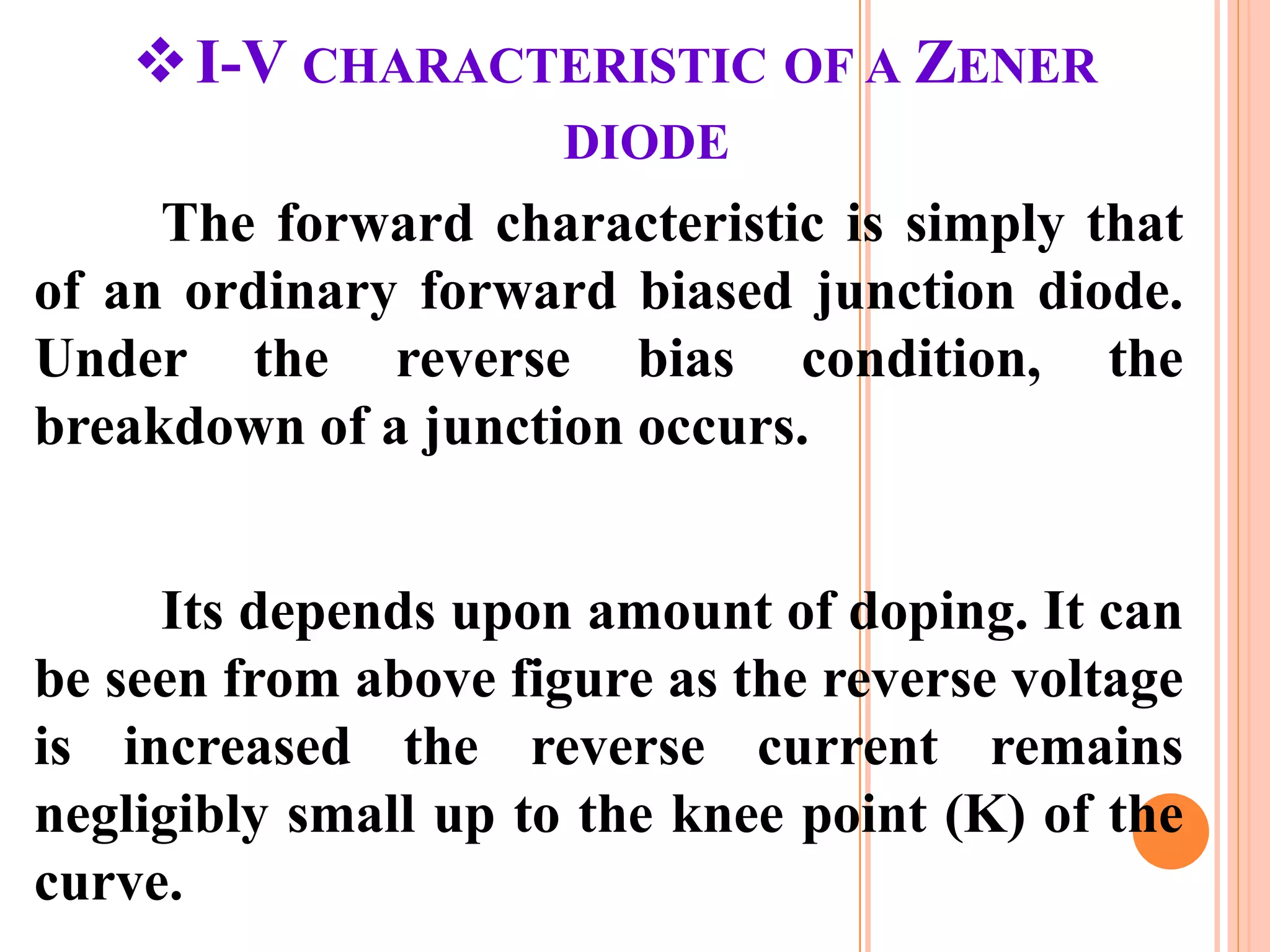
This document provides an overview of band theory of solids. It discusses effective mass of electrons in solids, the concept of holes, and the energy band structure of conductors, semiconductors, and insulators. Intrinsic and extrinsic semiconductors are described, along with p-type and n-type materials. Simple diode and Zener diode operation is summarized, including forward and reverse bias conditions.