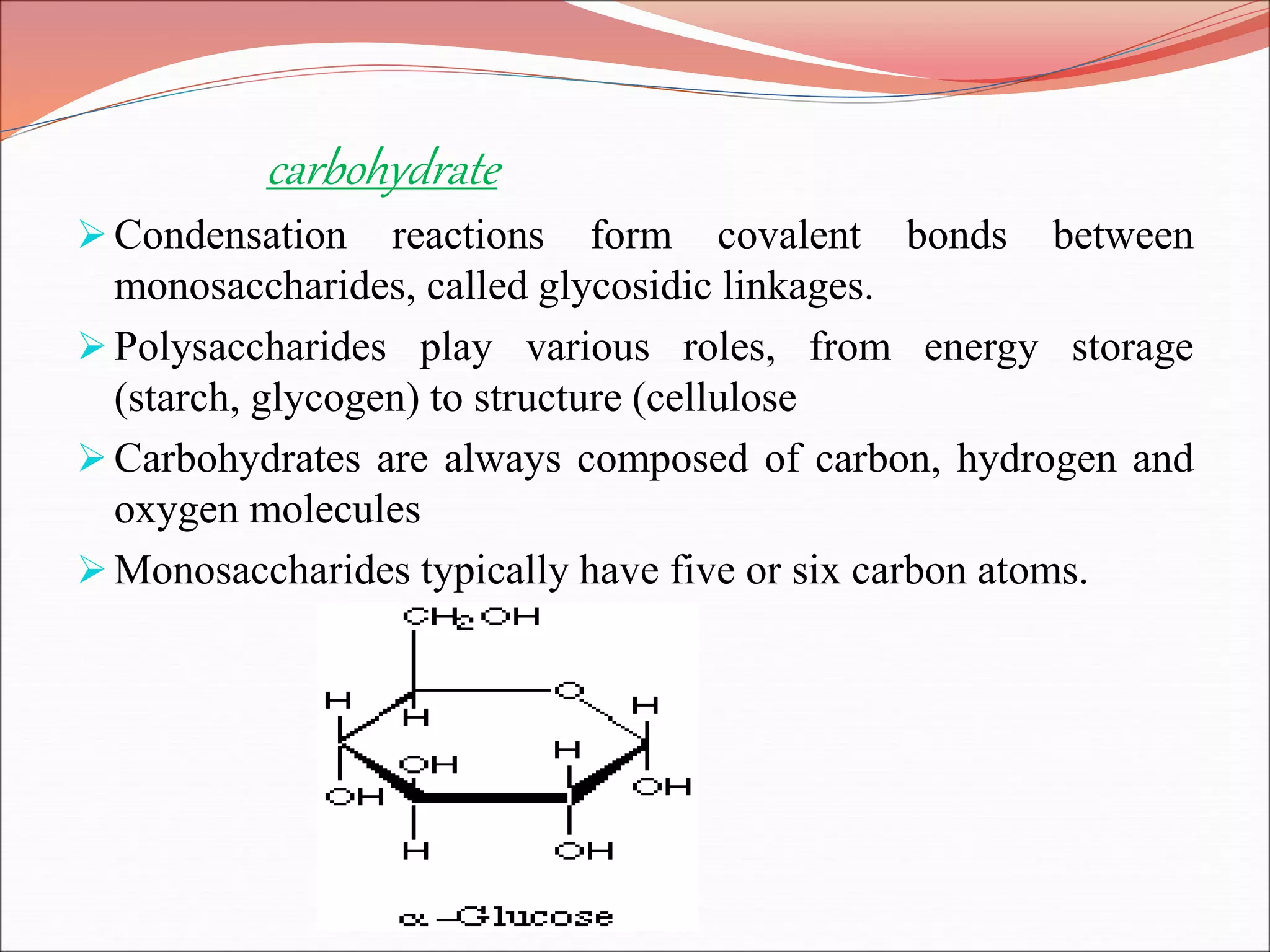
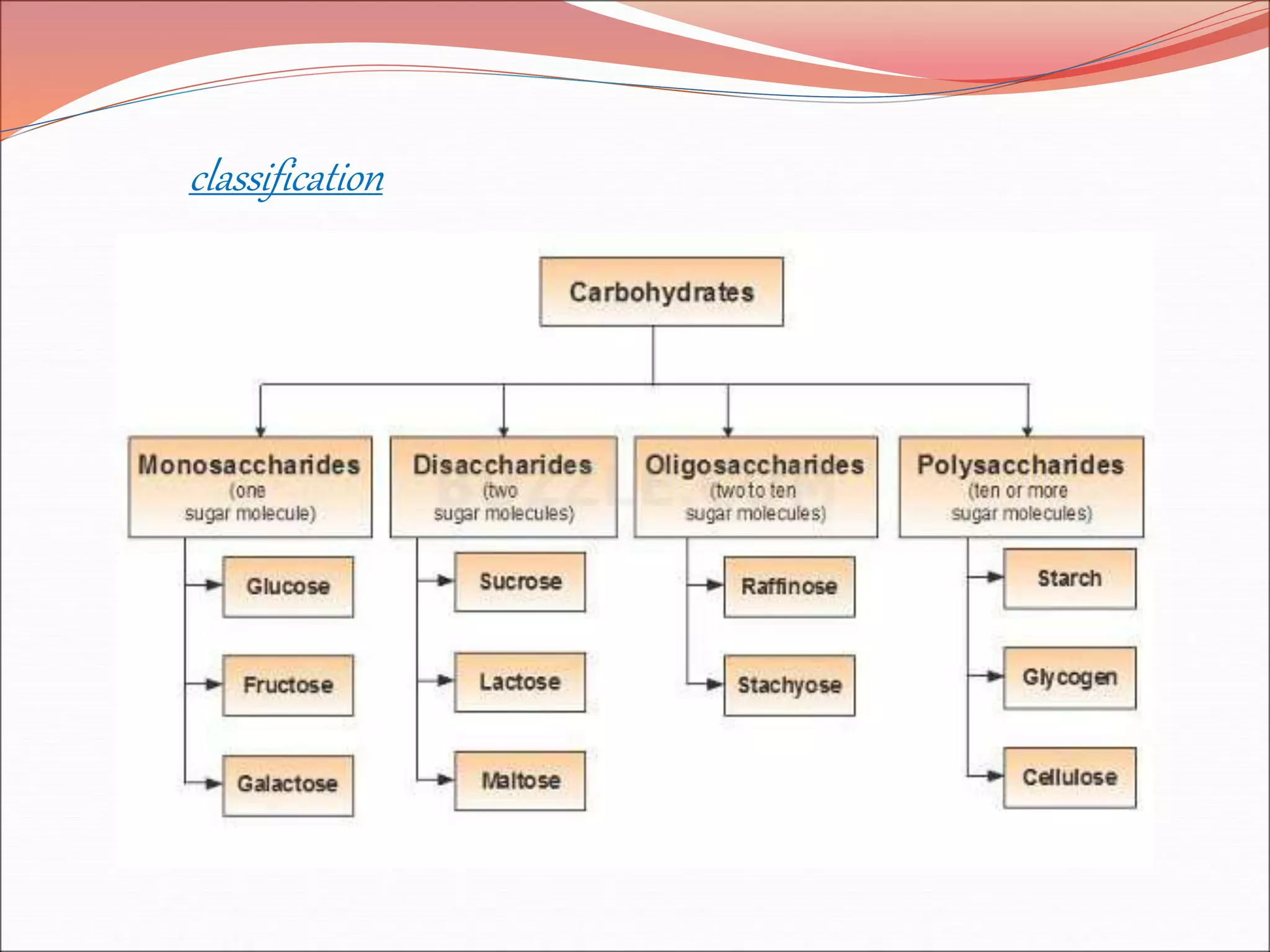
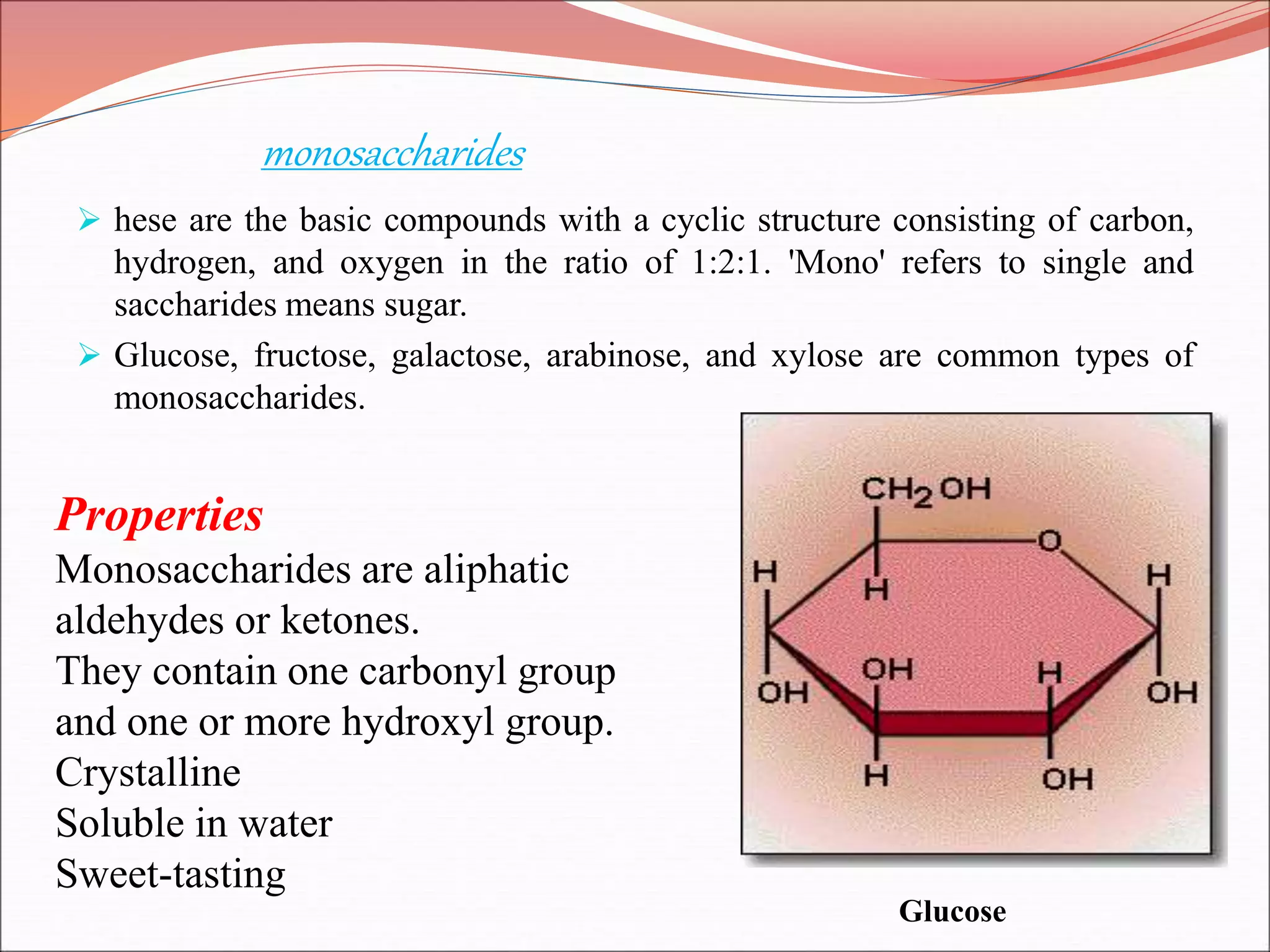
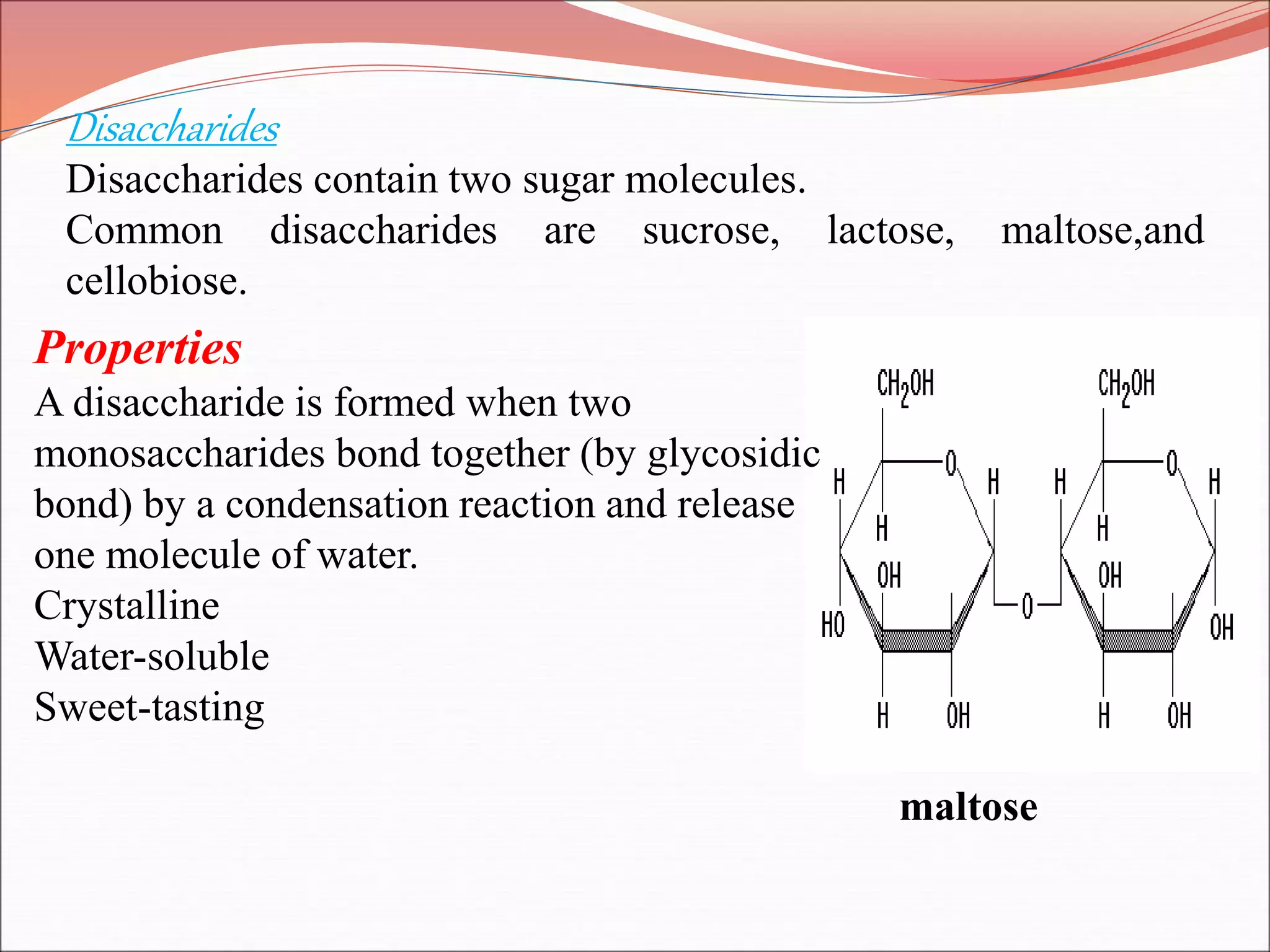
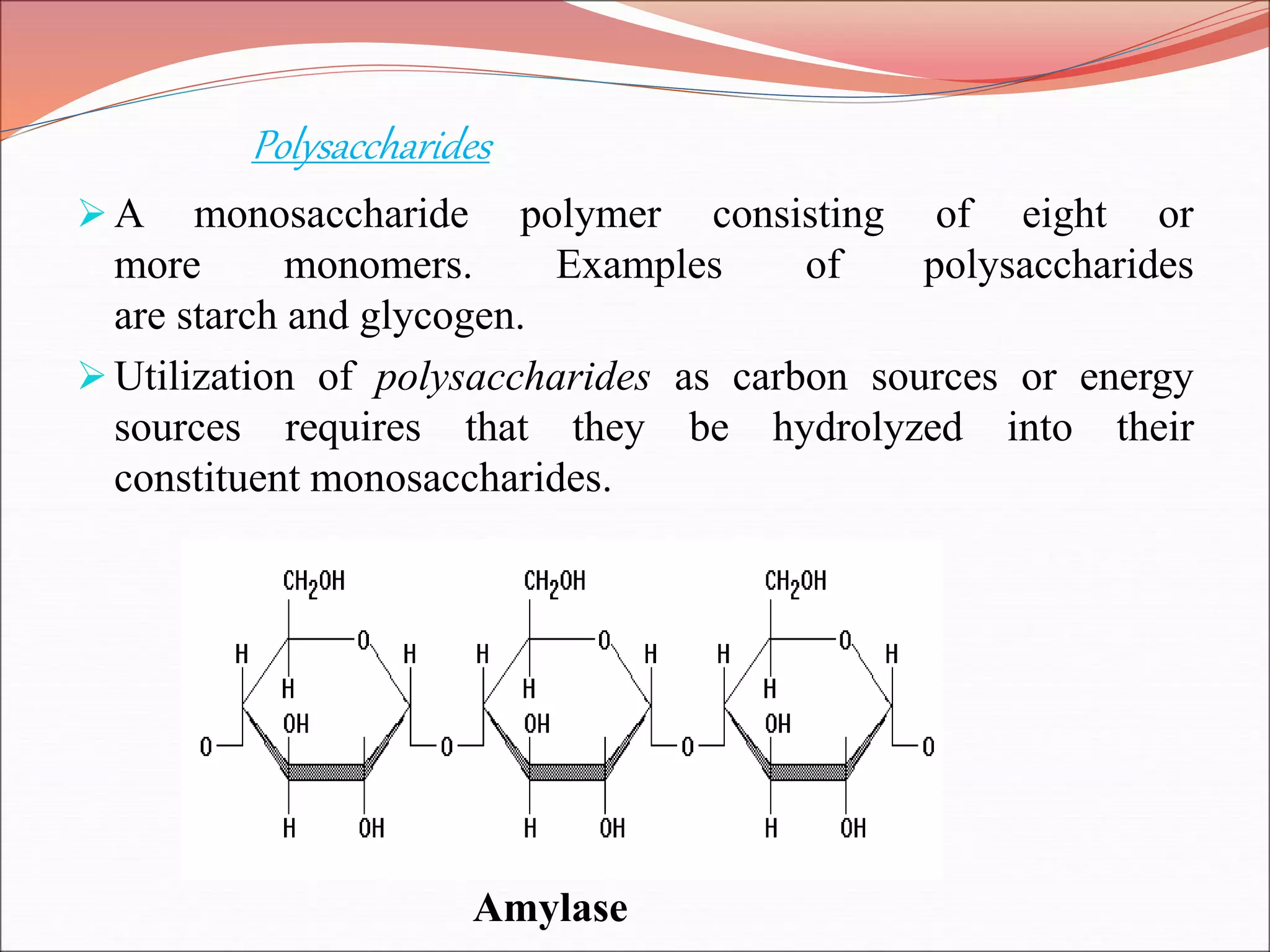
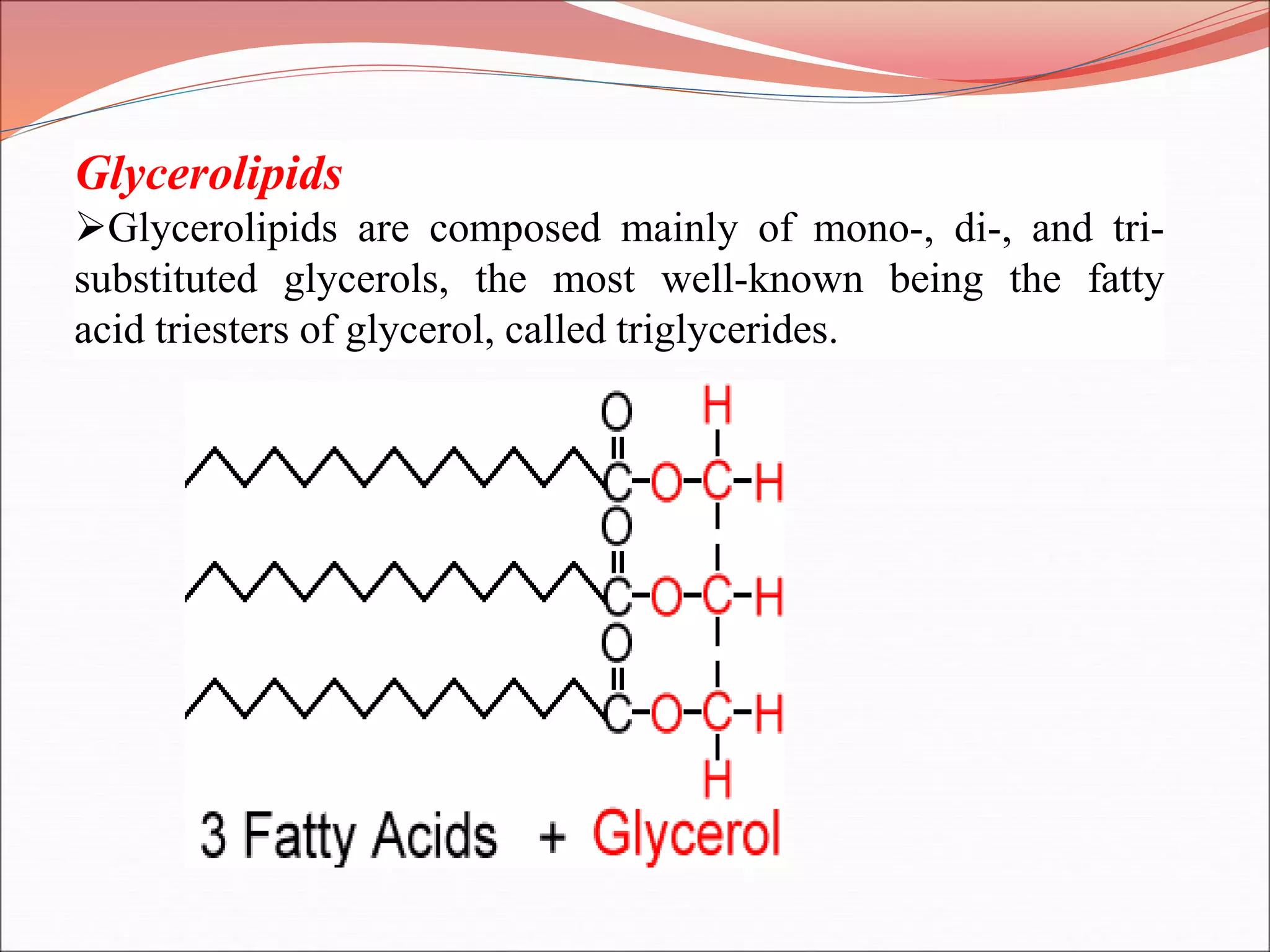
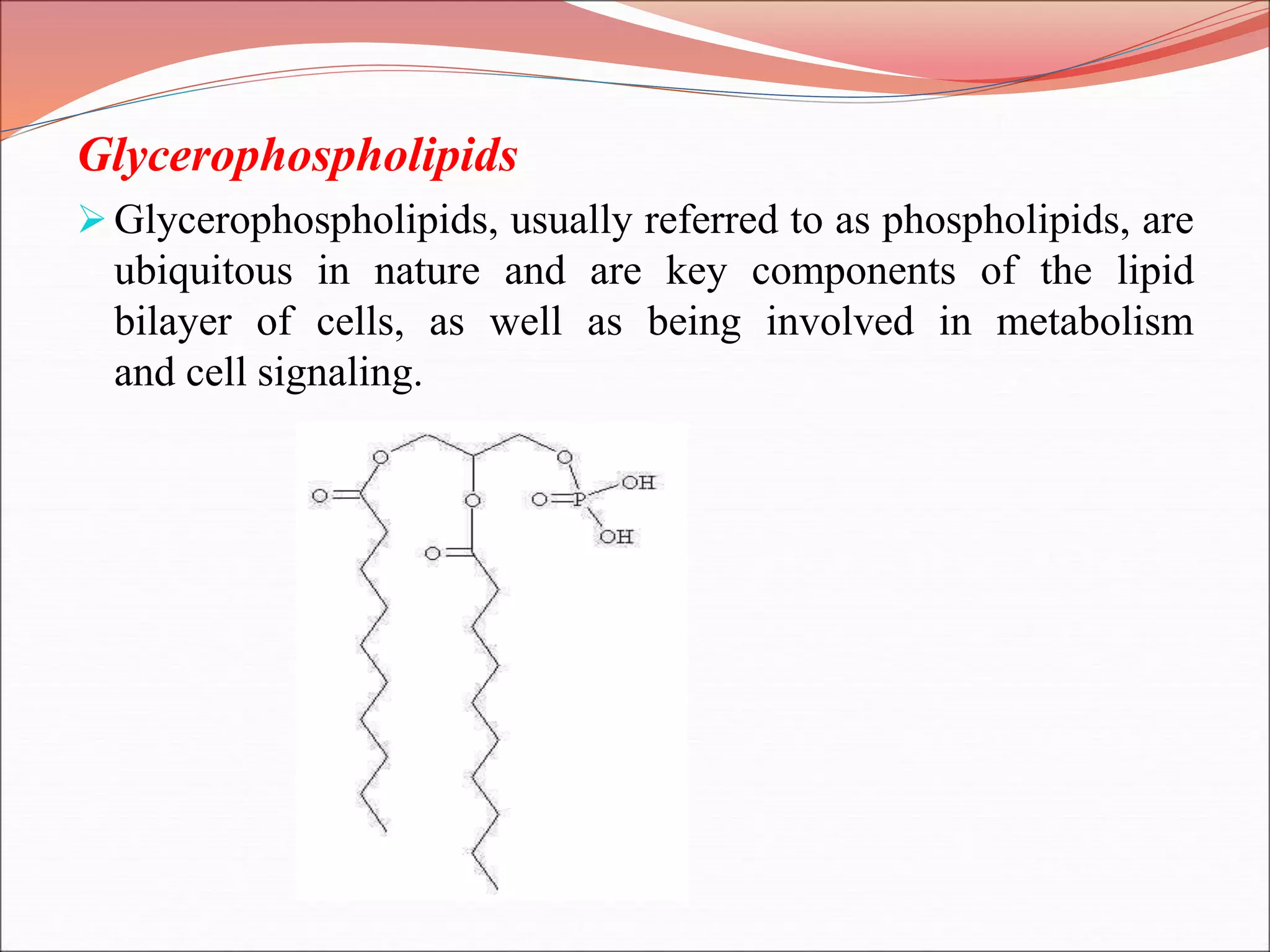
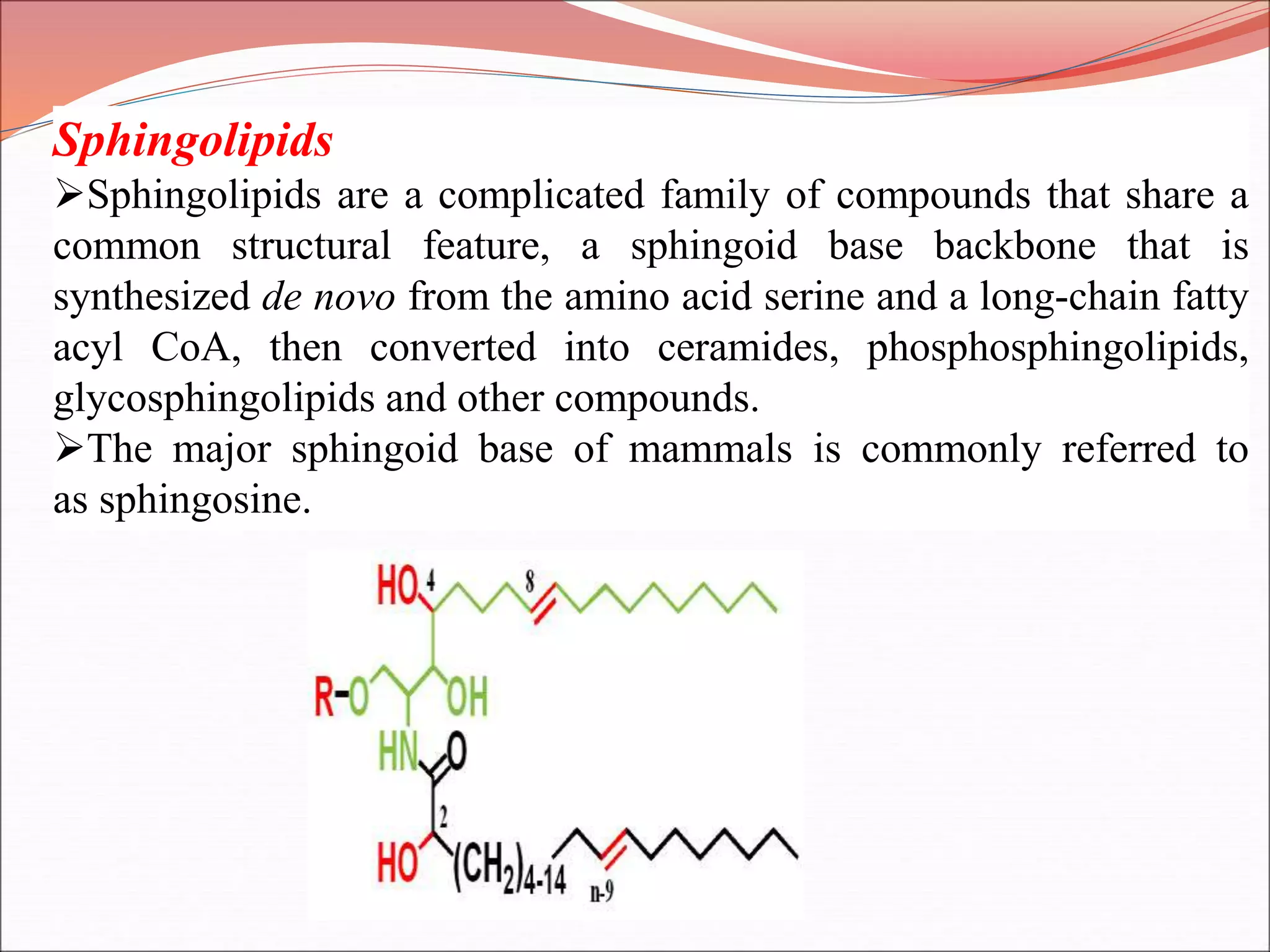
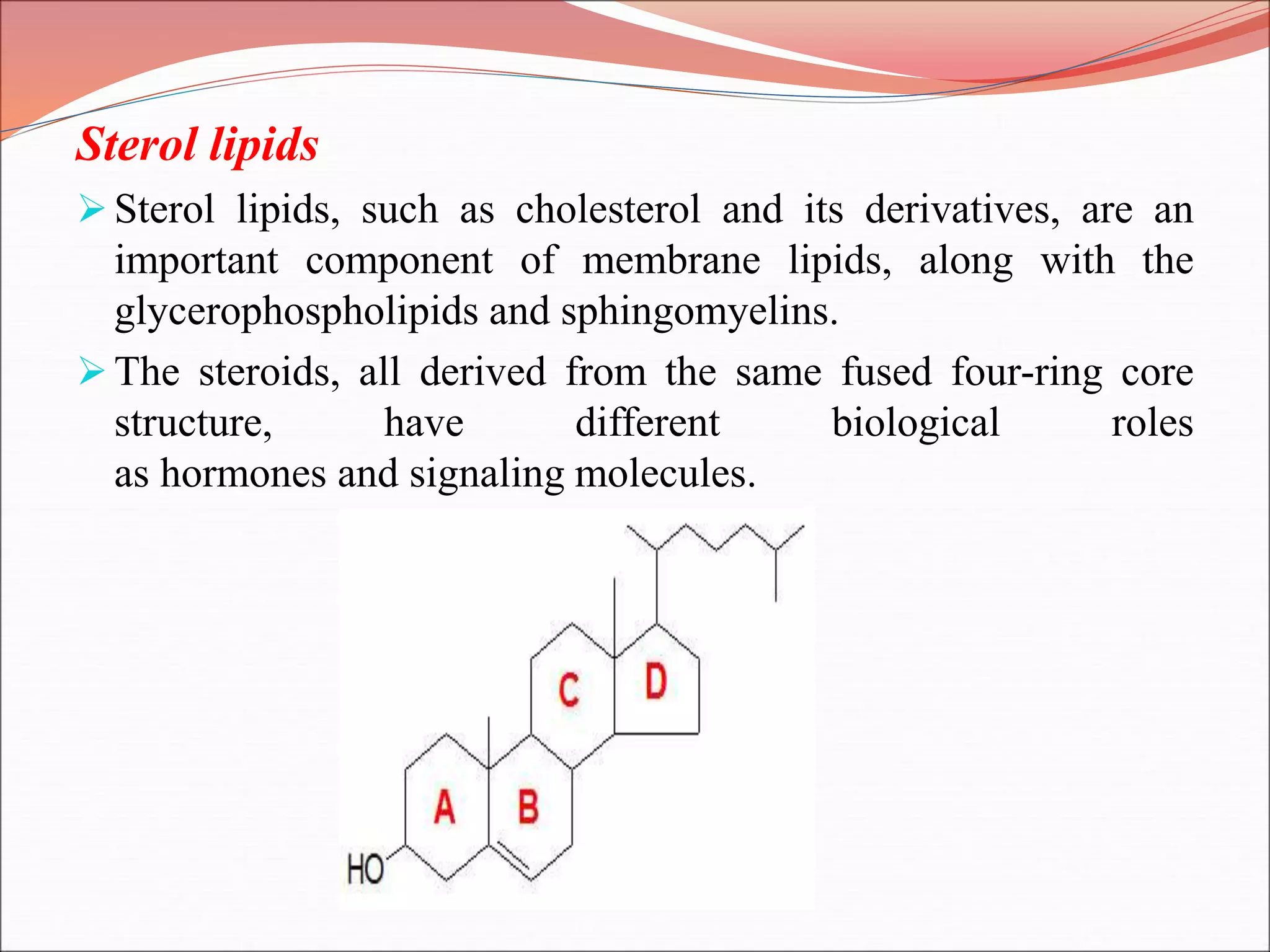
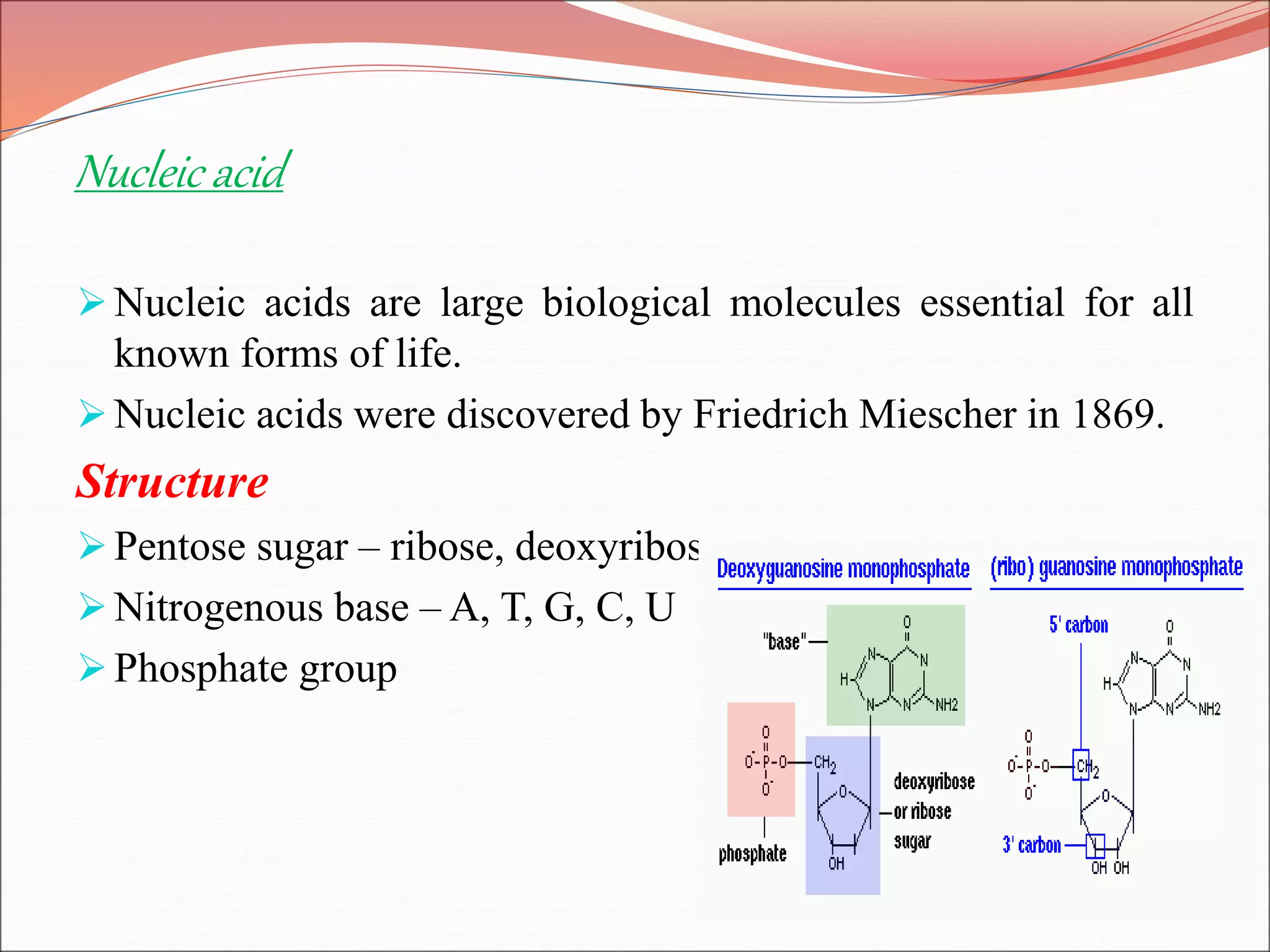
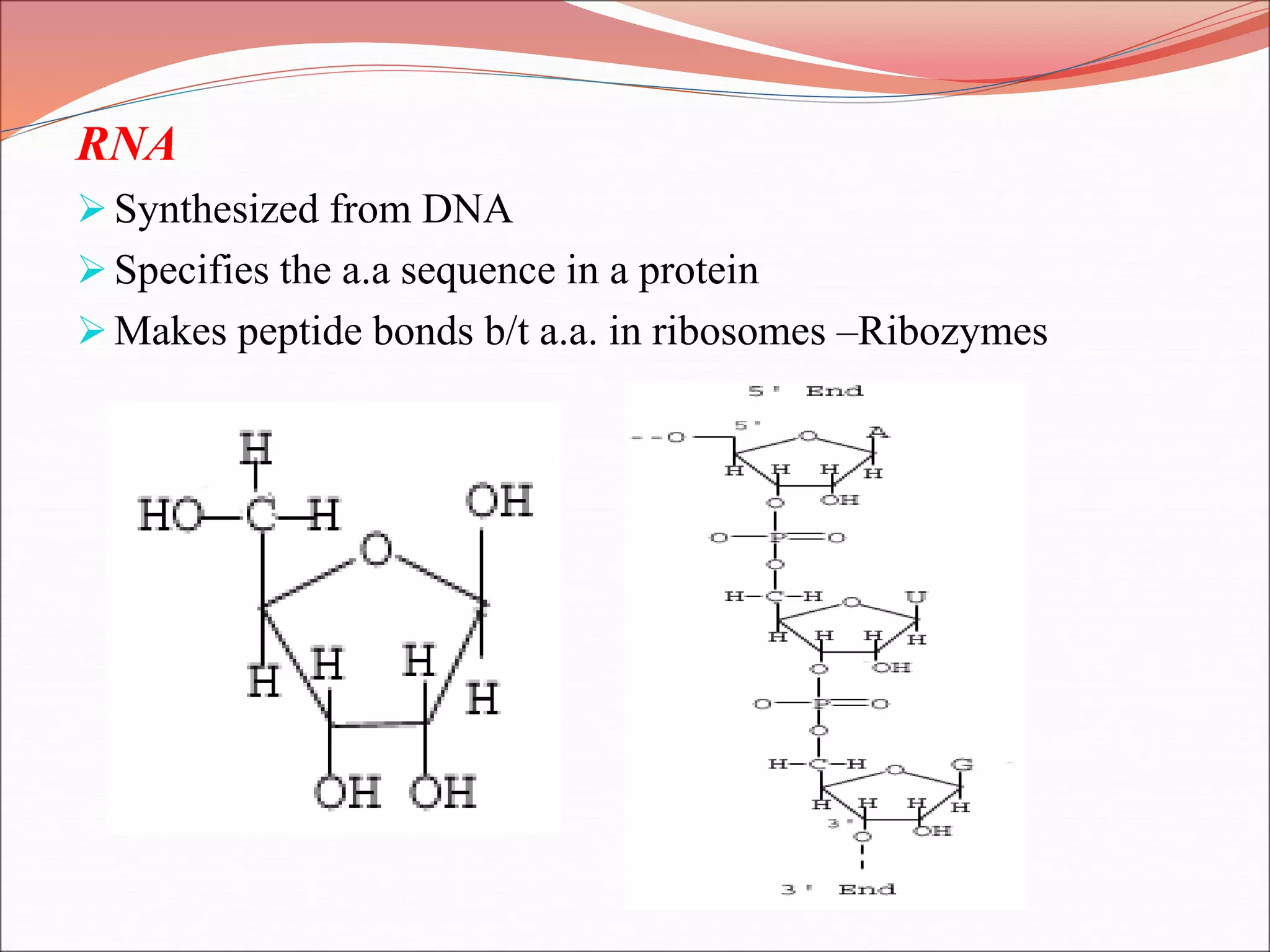
The document provides a comprehensive overview of macromolecules, detailing their definitions, types (proteins, carbohydrates, lipids, and nucleic acids), and their significant roles in biological processes. It covers the historical context of macromolecule research, structural properties, and the functions of each macromolecule type in living organisms. Additionally, it includes information on polymerization, protein structures, carbohydrate classifications, and the biological functions of nucleic acids.