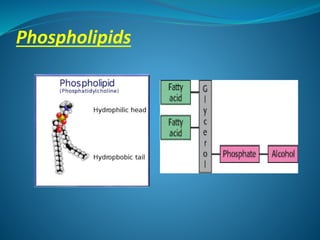
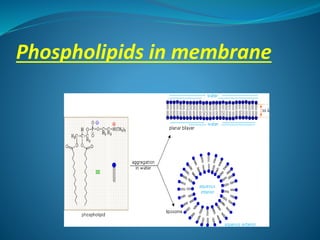
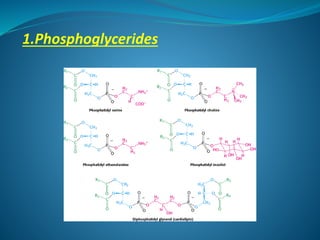
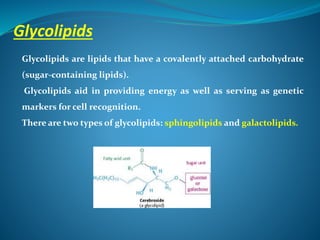
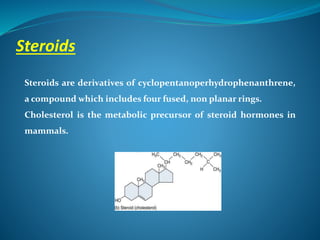
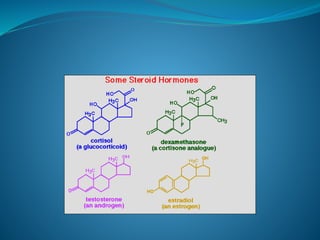
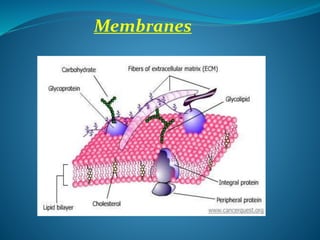
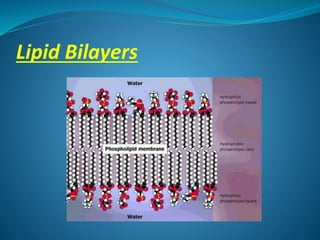
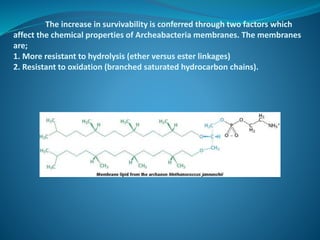
The document discusses structural lipids, focusing on their role in biological membranes, types such as phospholipids, glycolipids, and cholesterol, and their historical significance. It highlights the amphipathic nature of lipids, the structure of lipid bilayers, and differences between archaeal and bacterial membranes. The conclusion emphasizes the function of lipids in membrane permeability and fluidity.