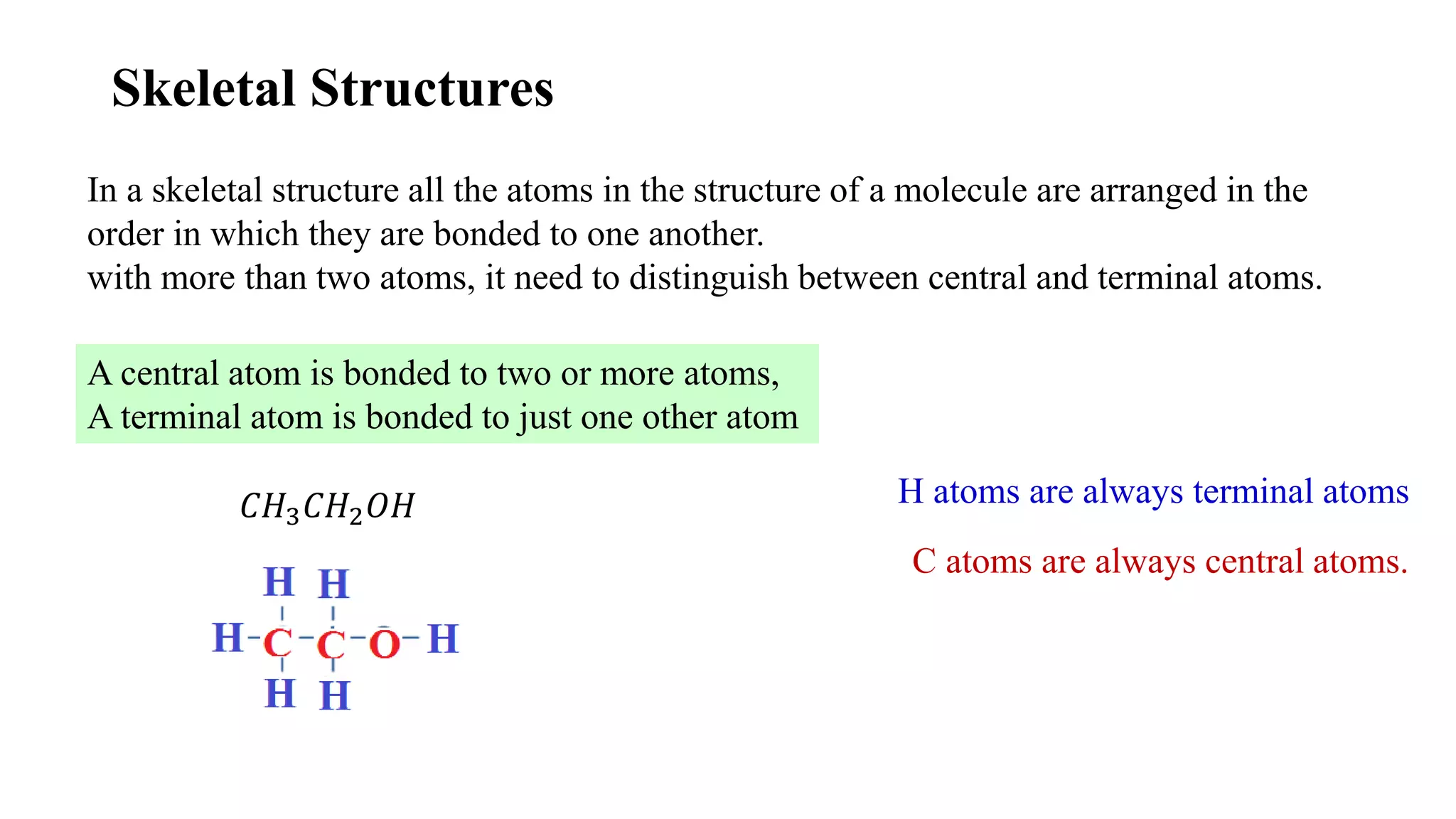
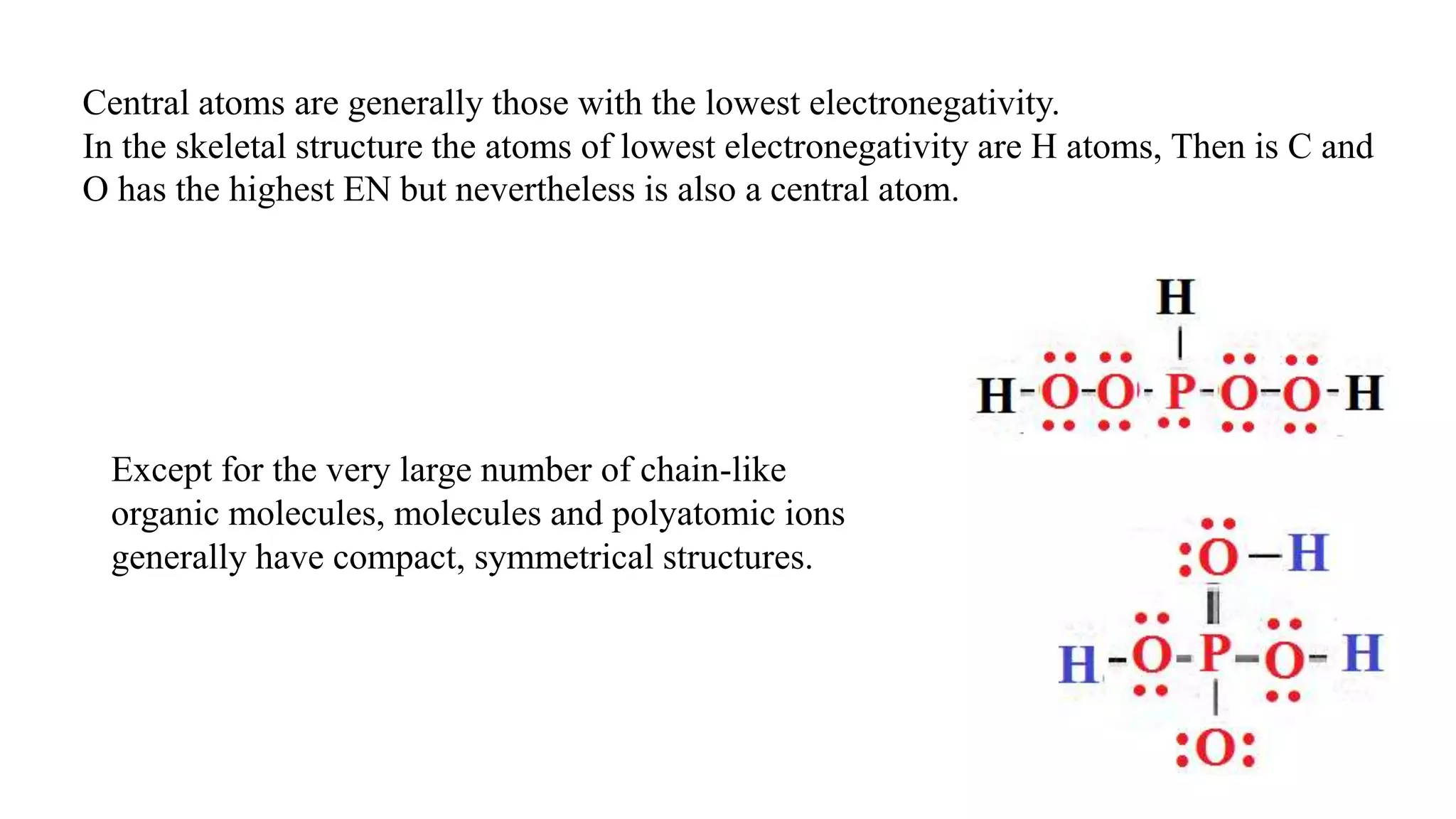
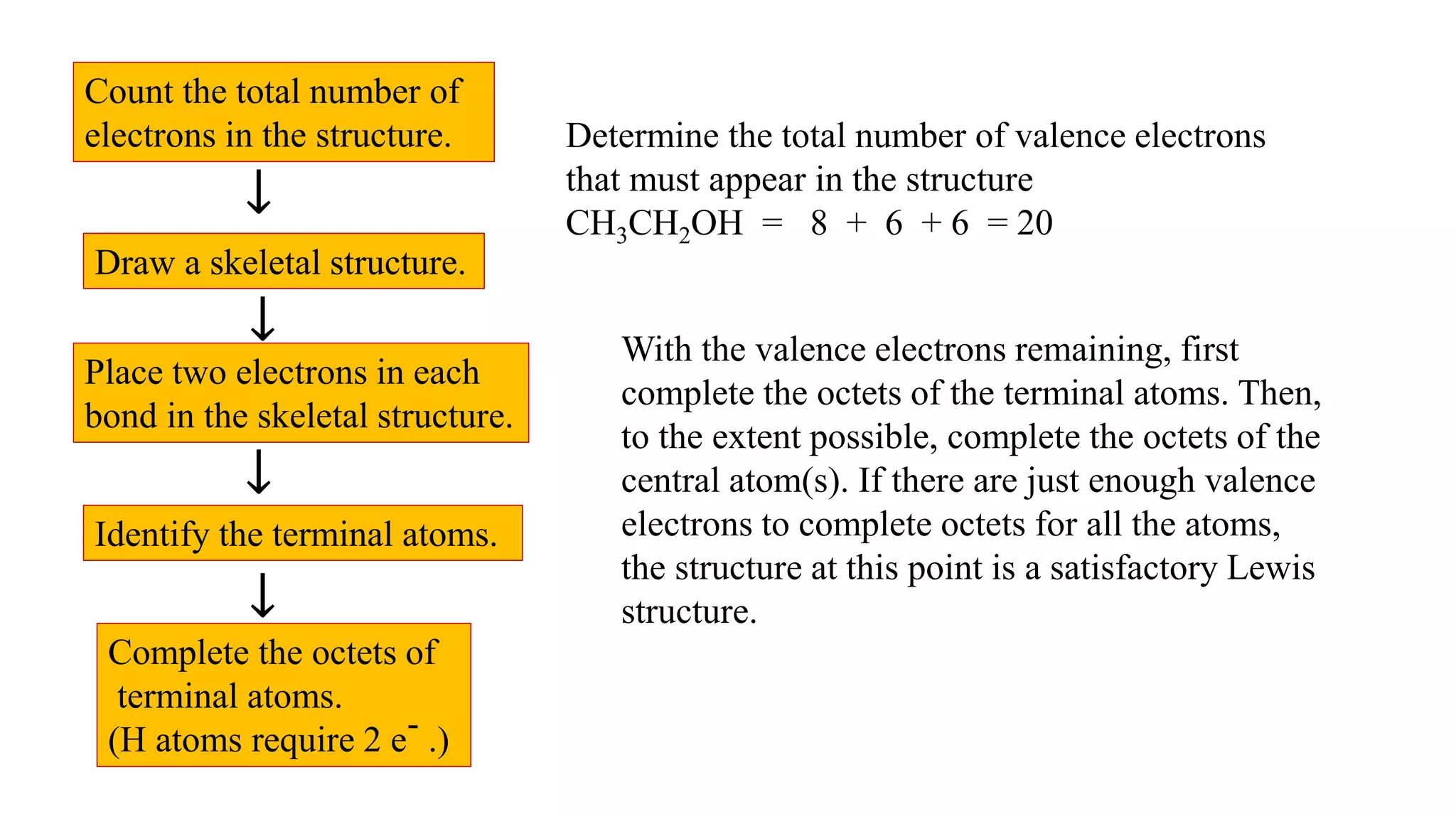
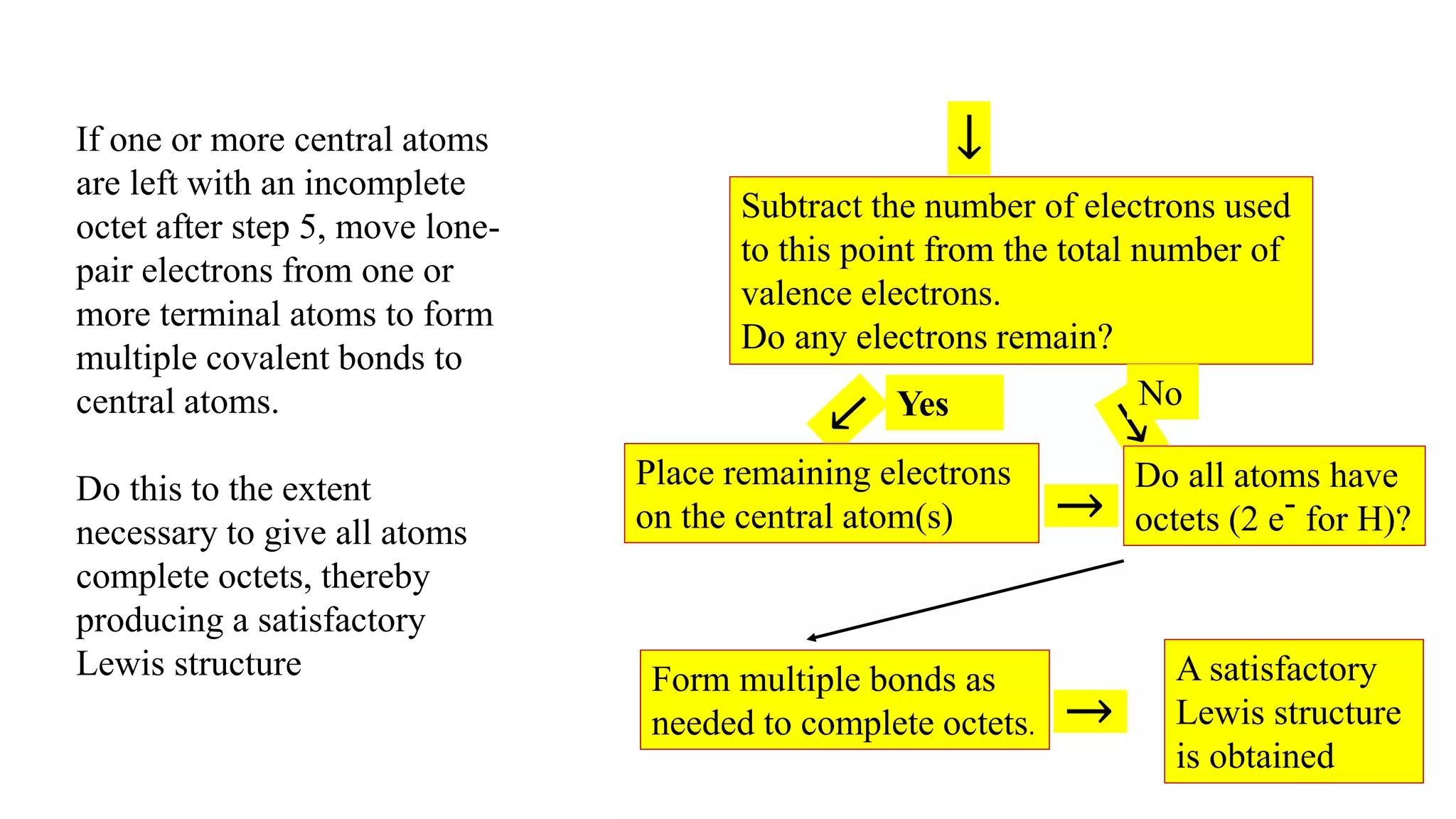
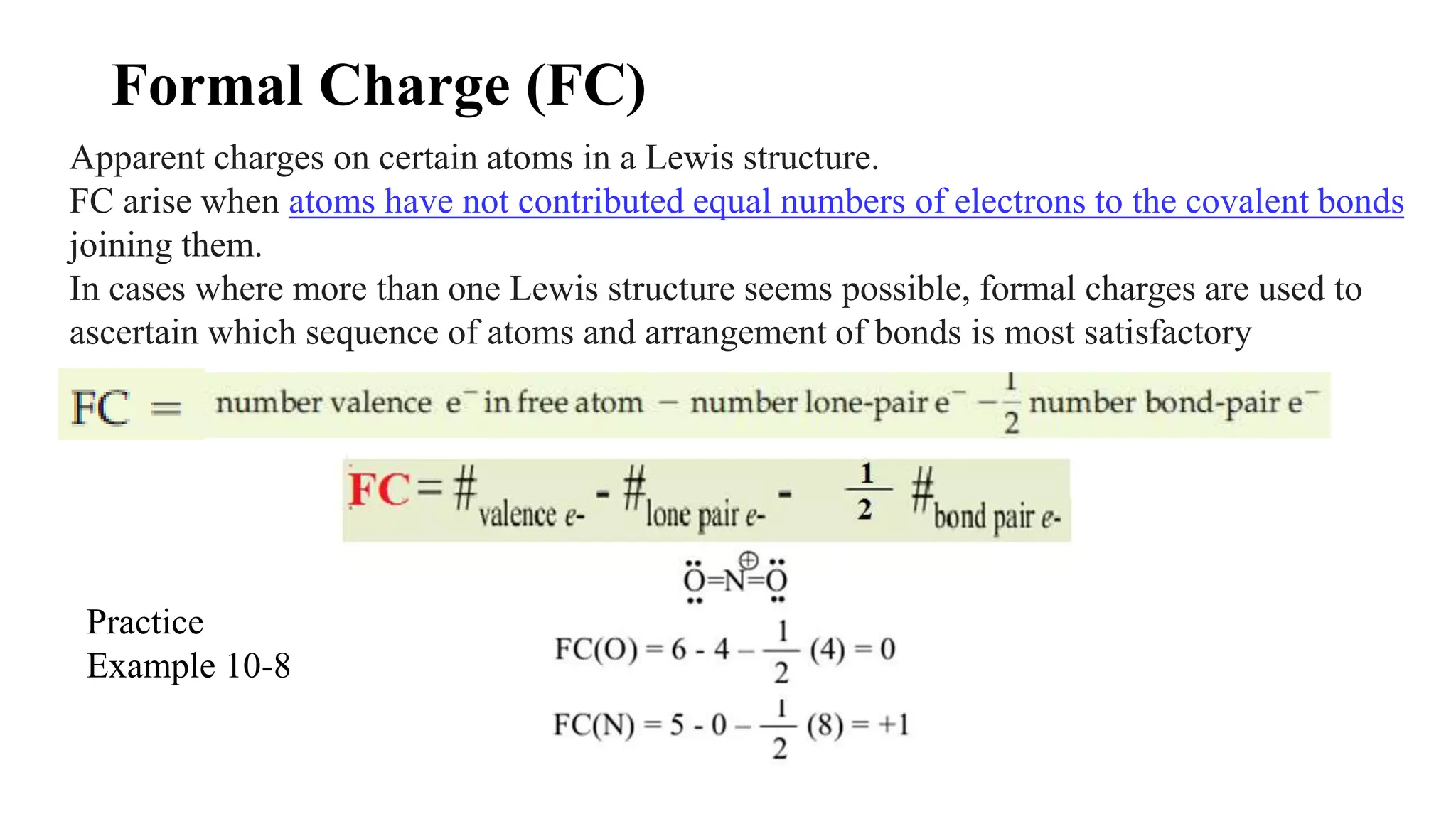
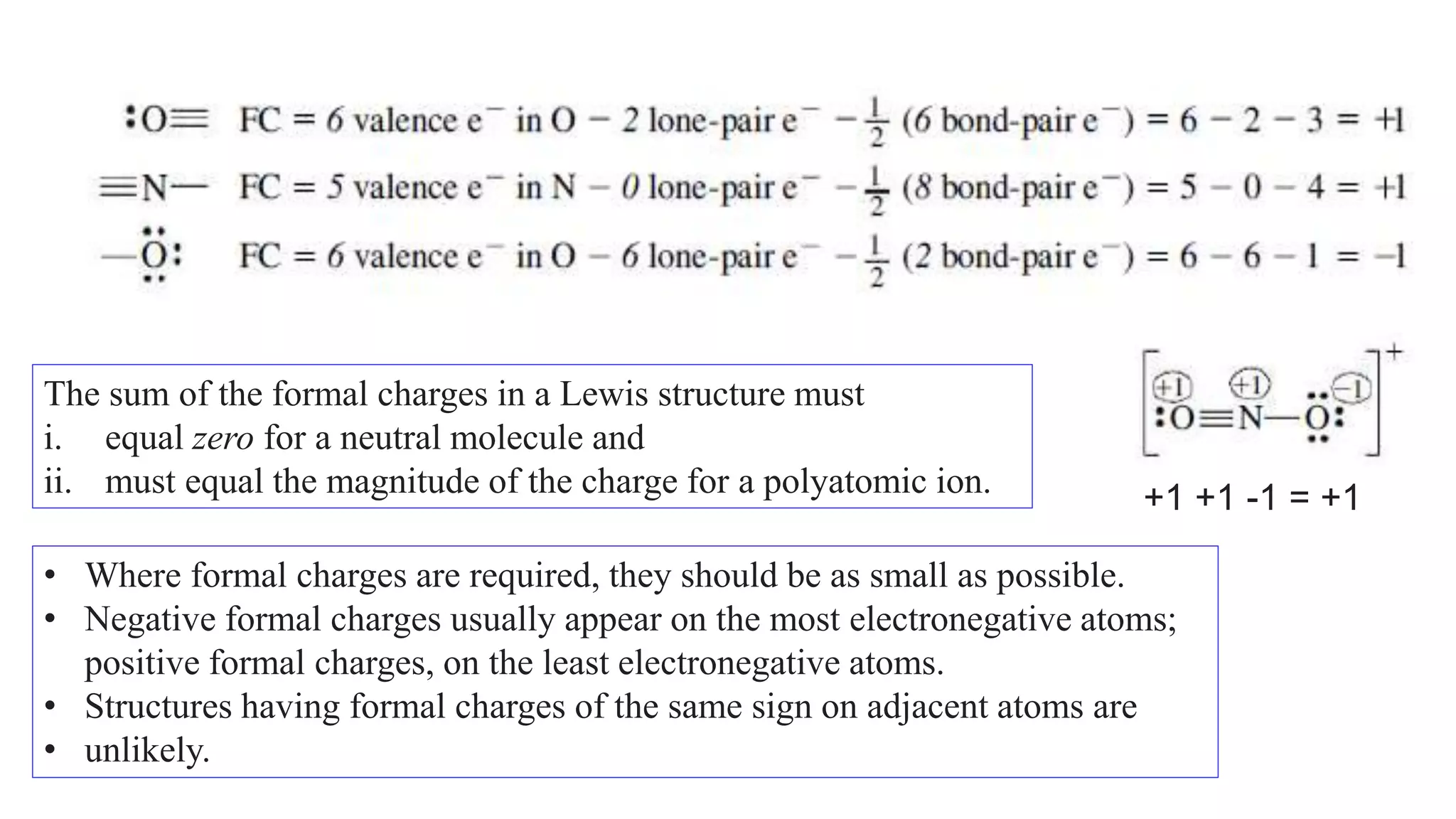
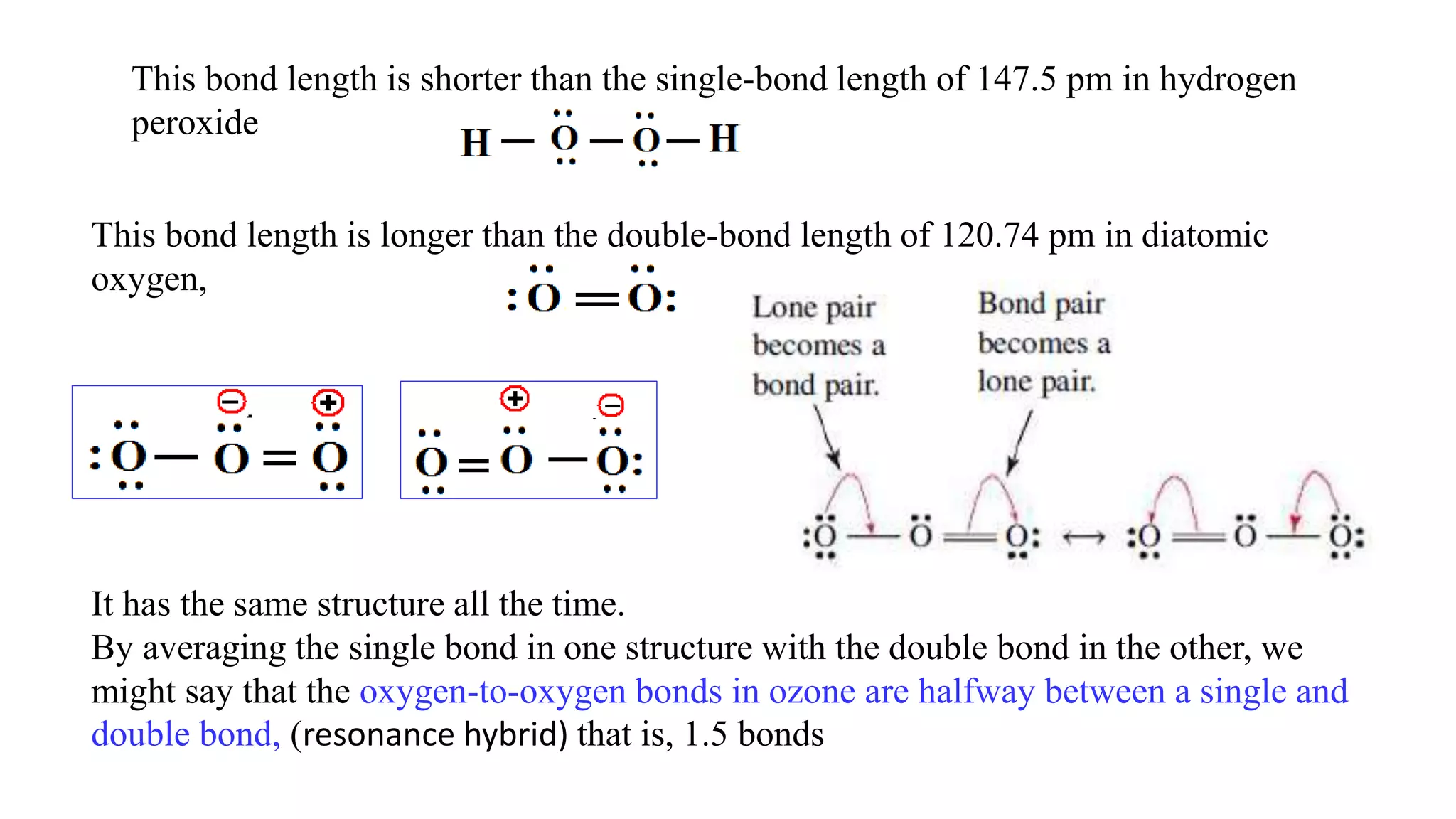
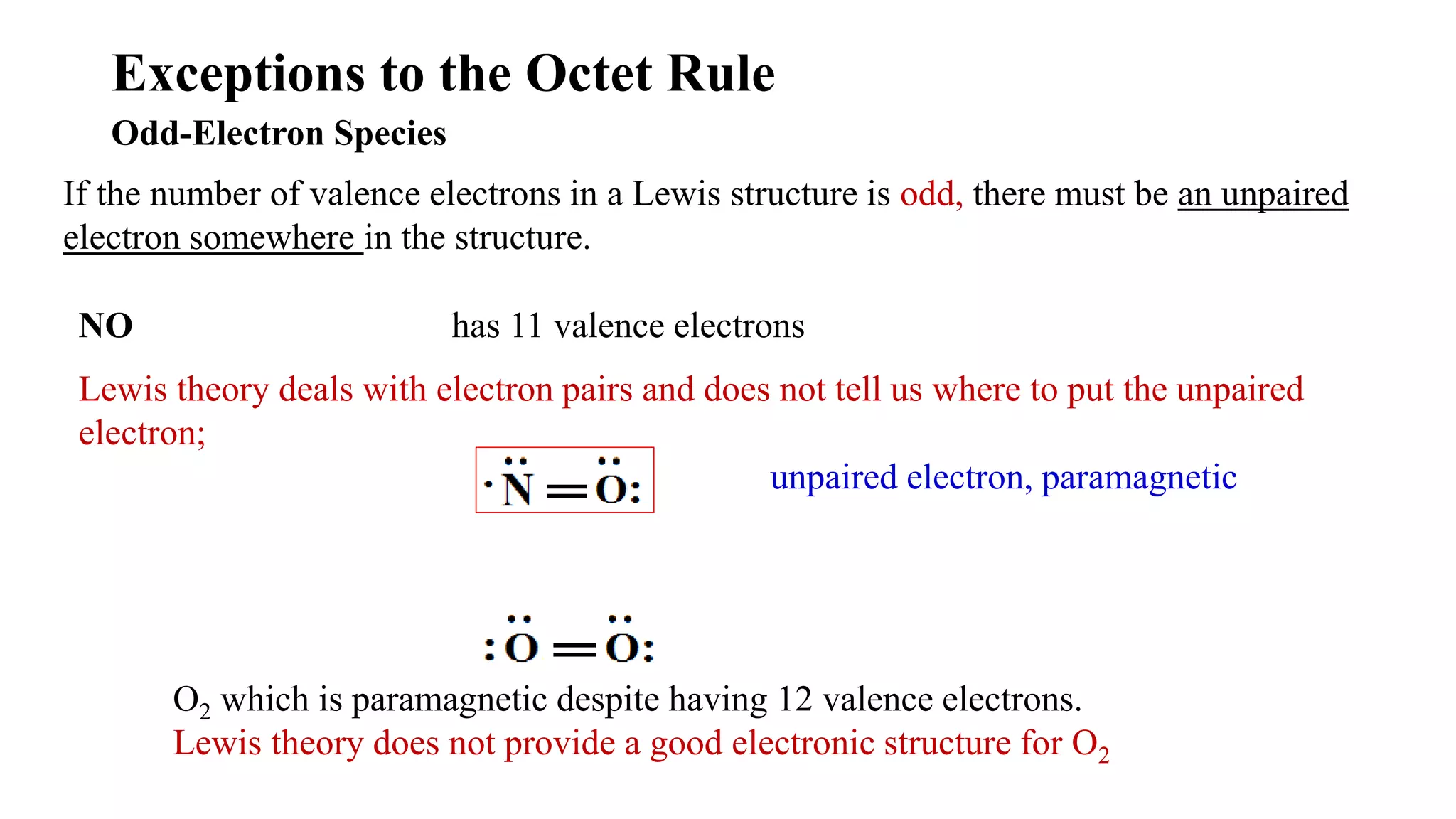
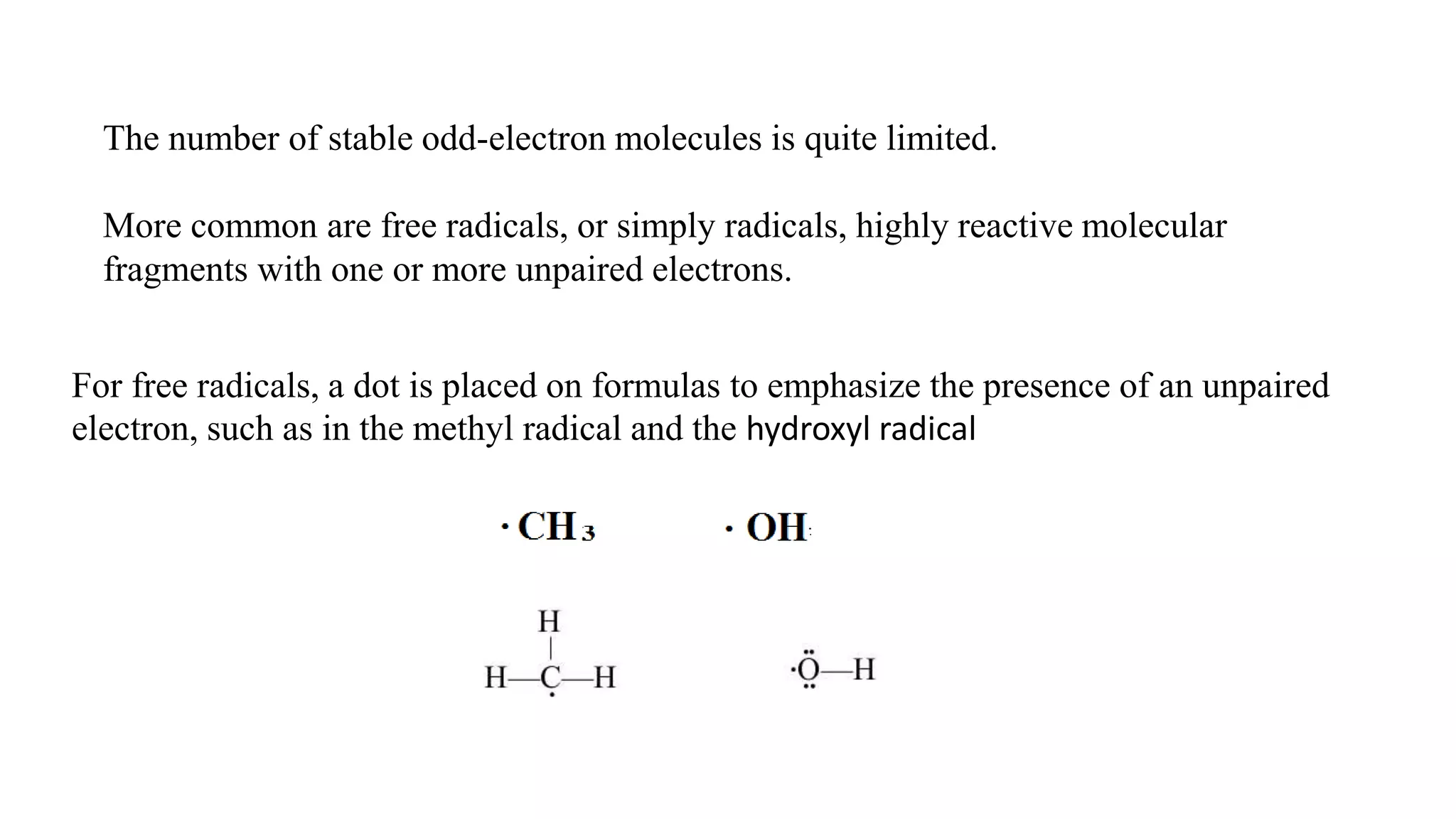
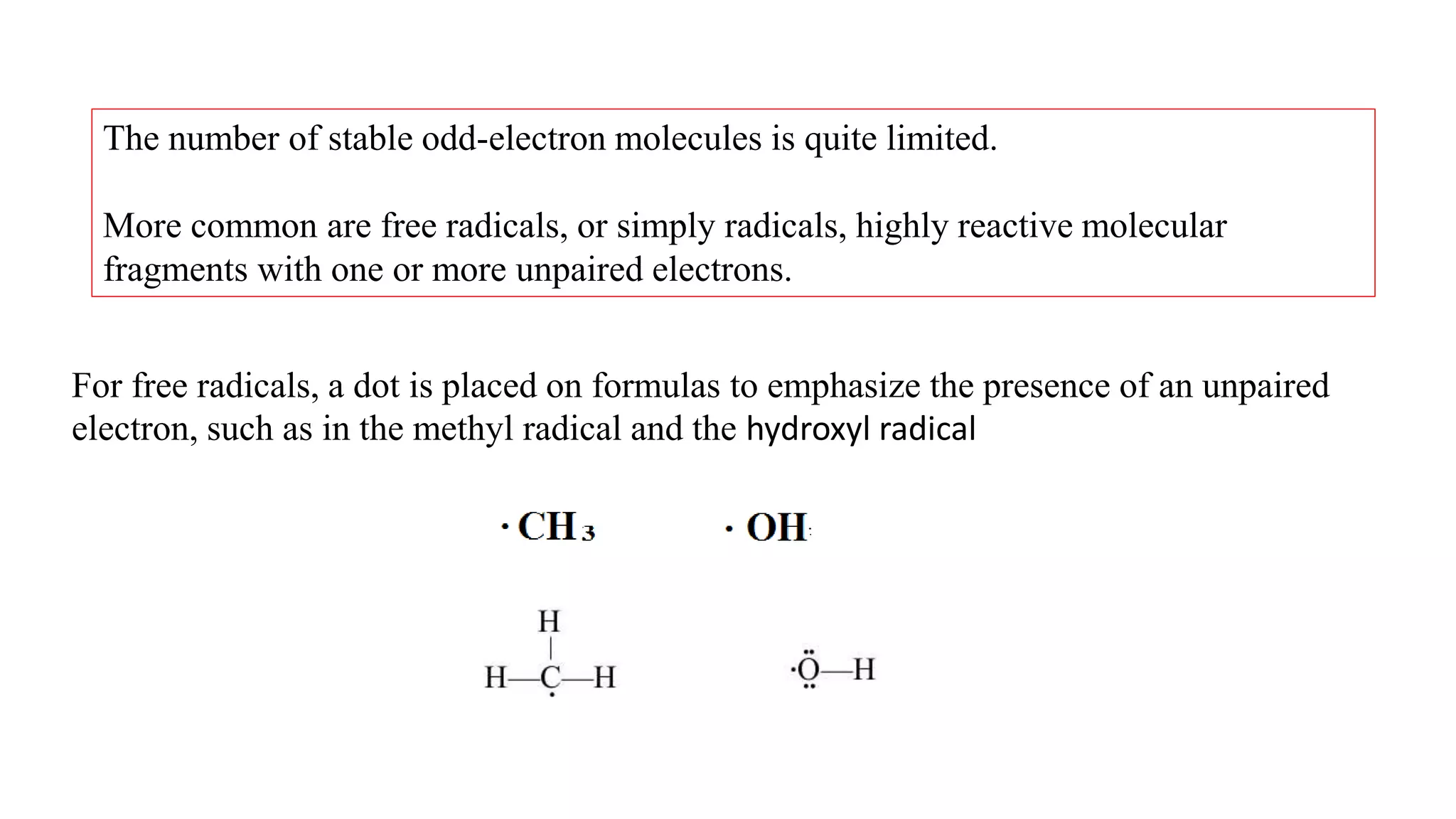
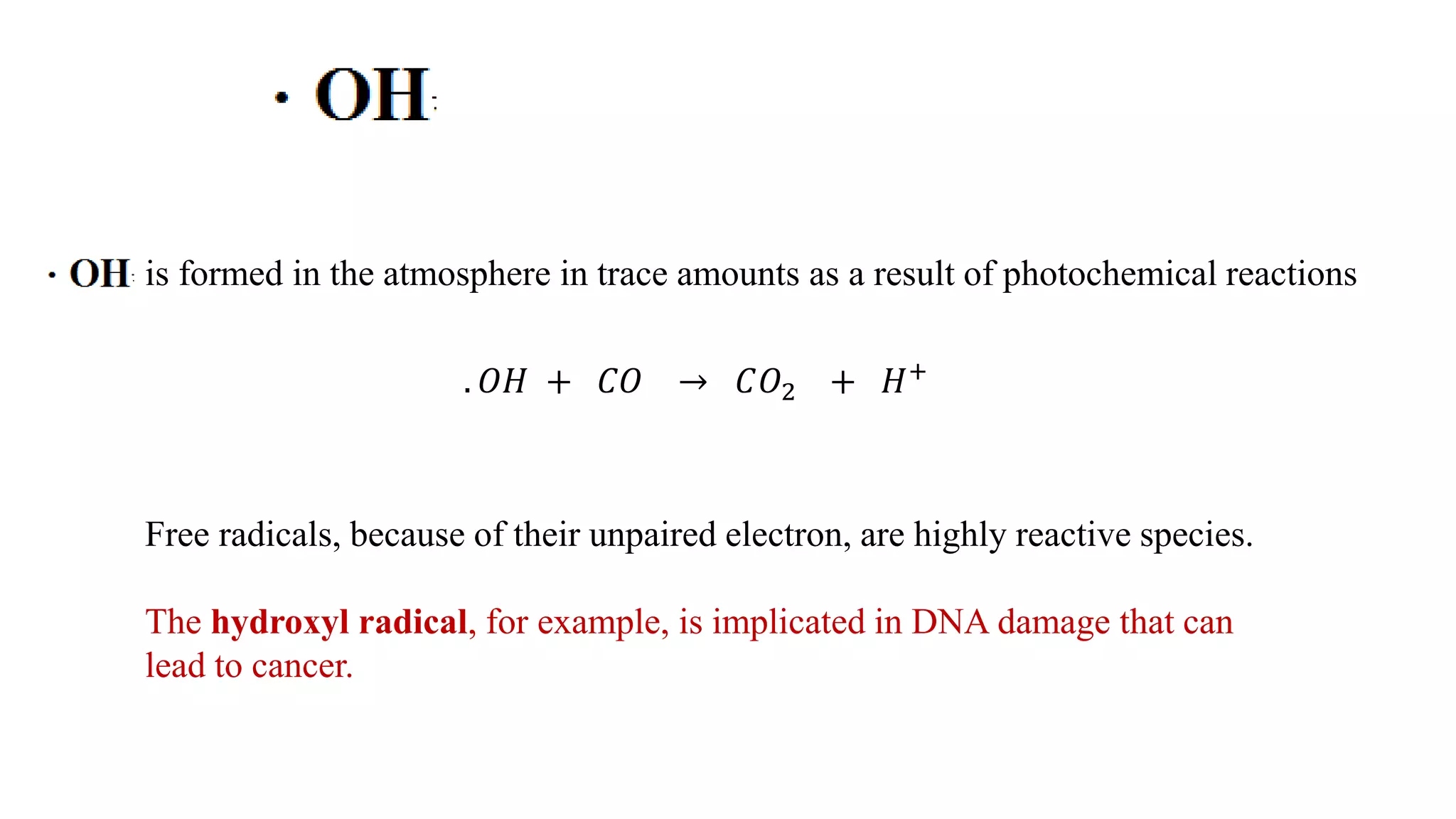
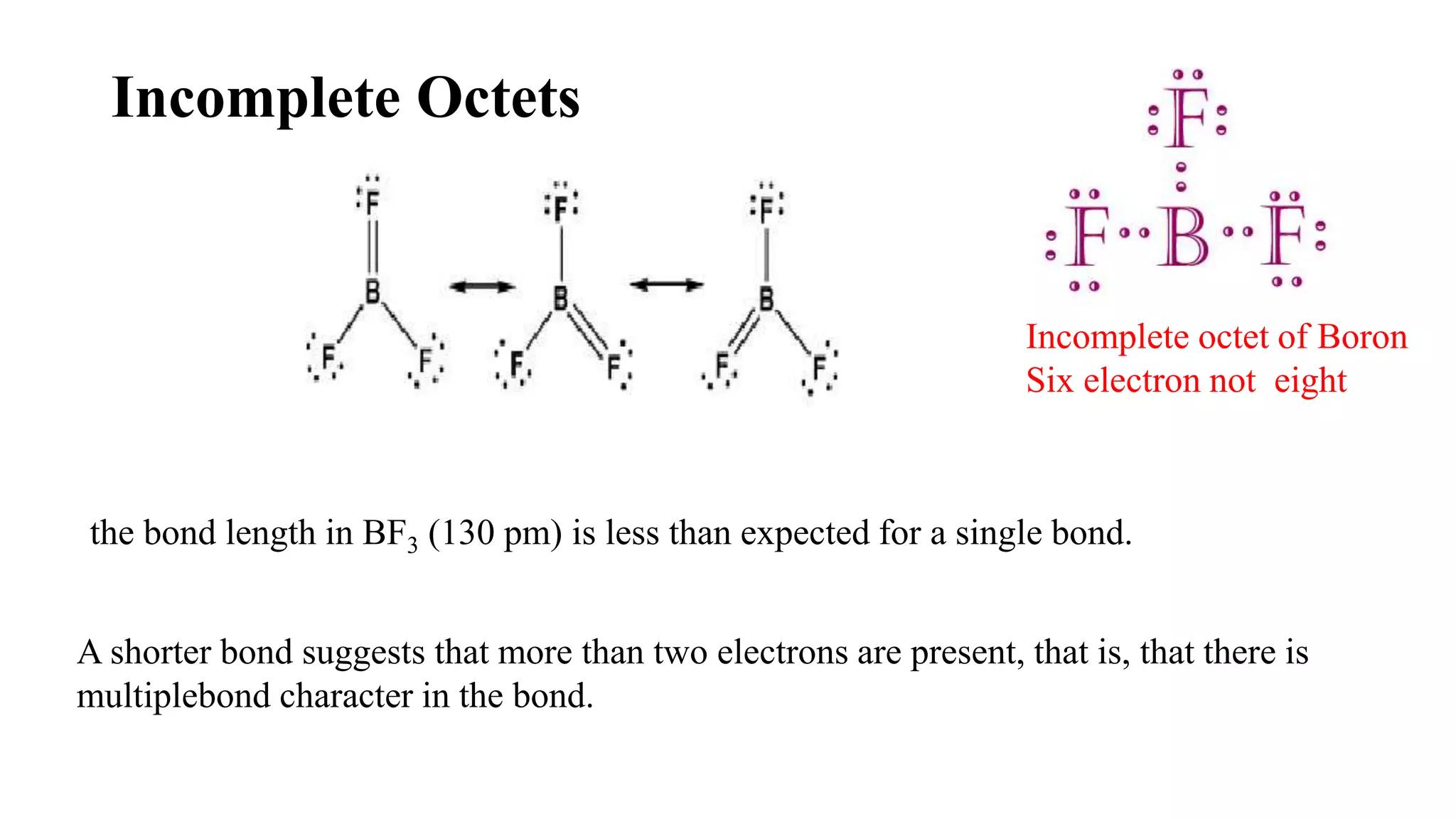
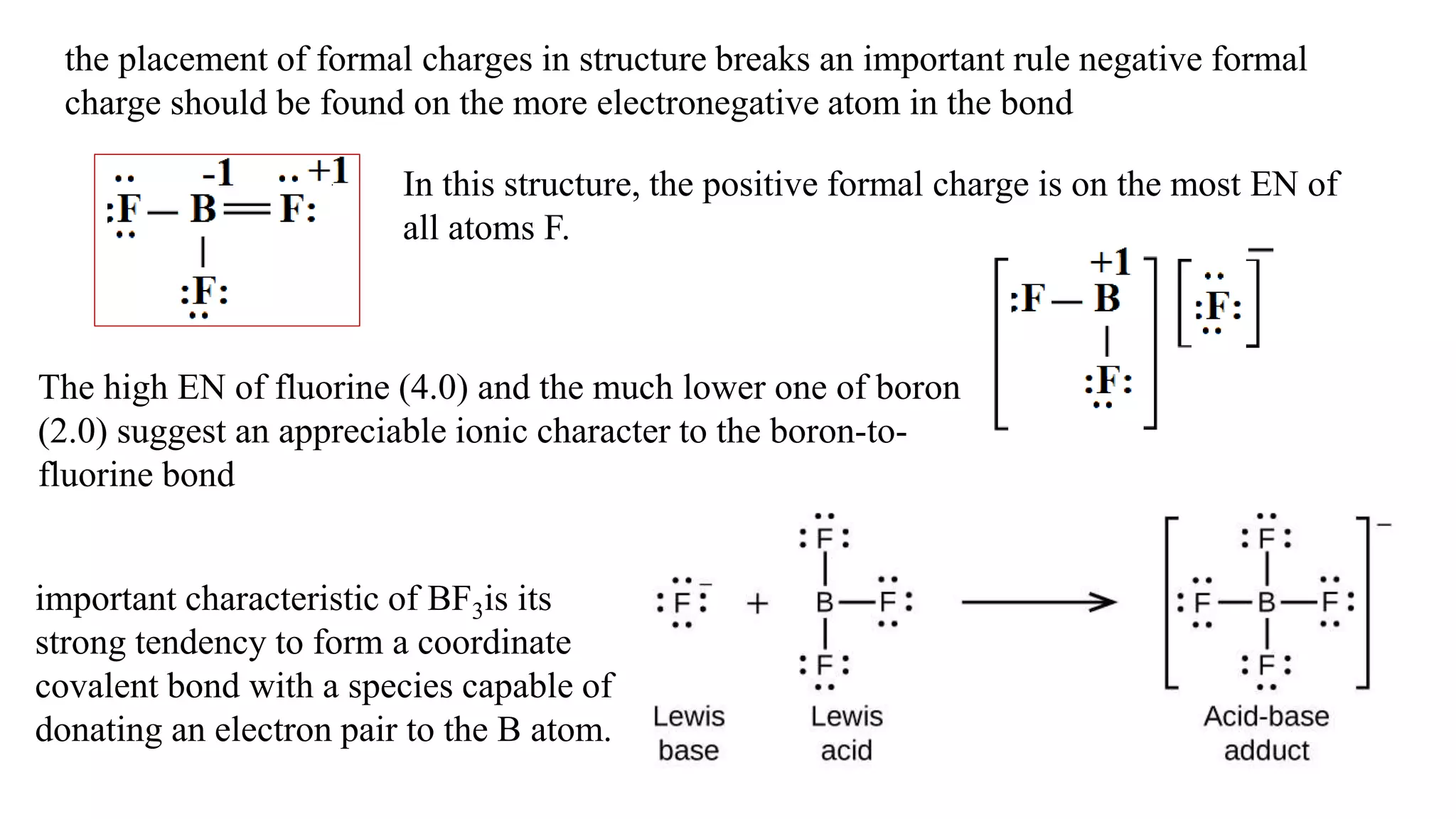
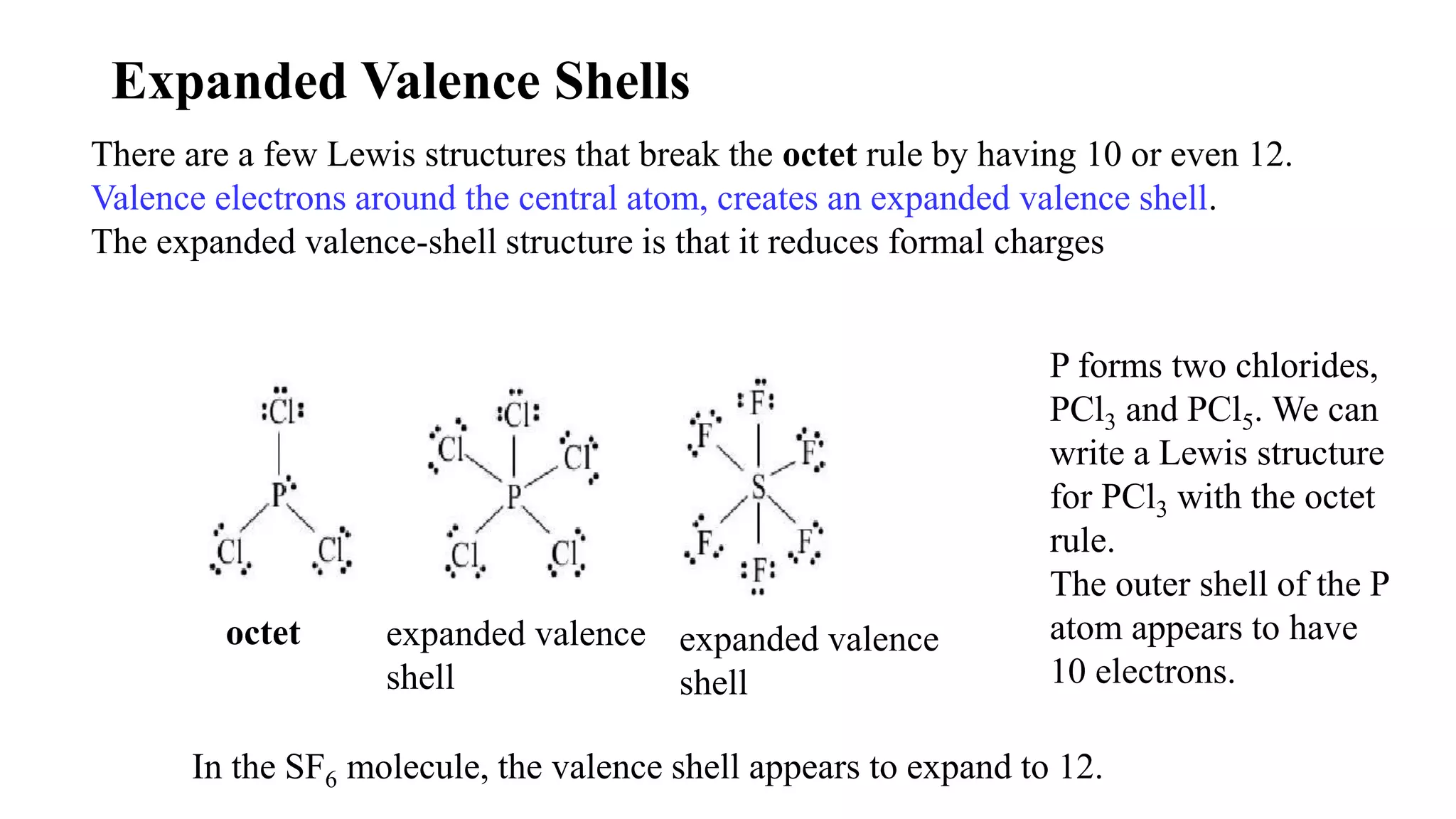
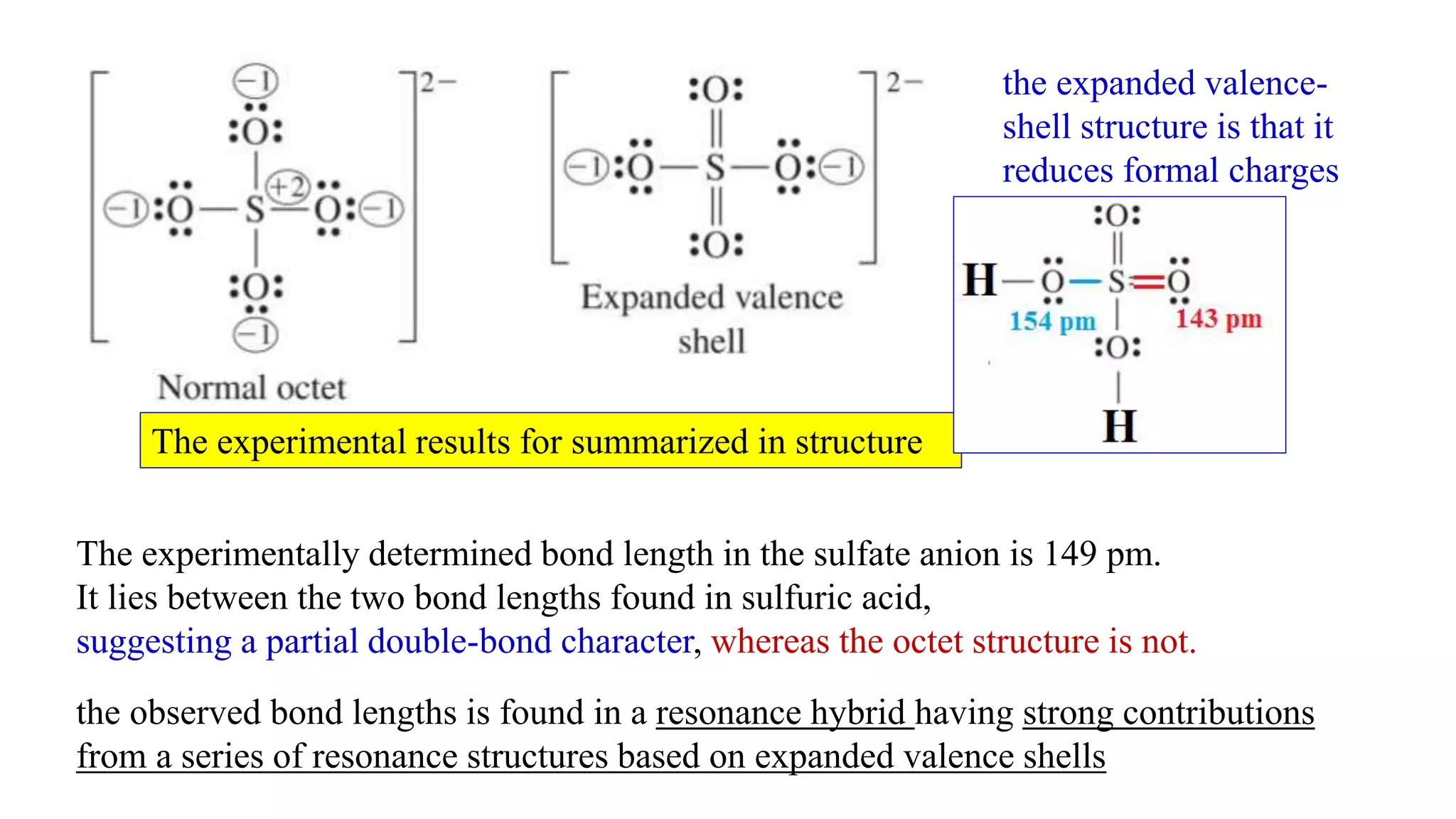
The document discusses skeletal structures and valence shells, detailing how to construct Lewis structures by identifying central and terminal atoms and ensuring that all atoms achieve complete octets. It introduces concepts such as formal charges, exceptions to the octet rule, and the presence of free radicals, emphasizing their reactivity and implications in chemistry. The text also touches on expanded valence shells and resonance structures, providing insights into bond lengths and the stability of various molecular arrangements.