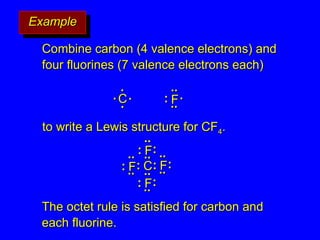
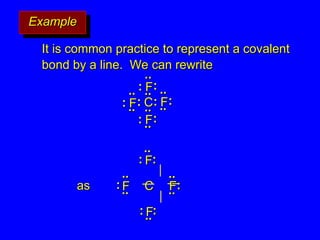
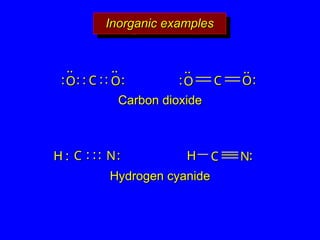
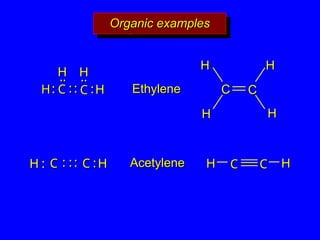
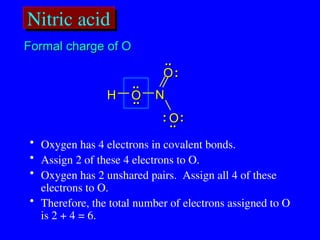
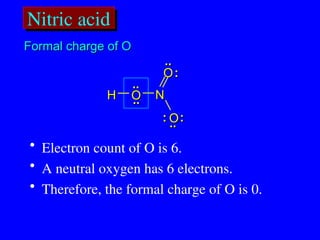
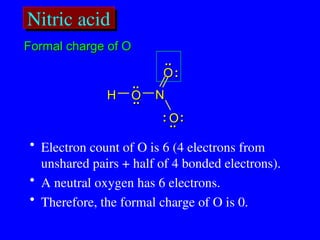
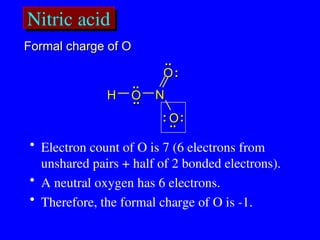
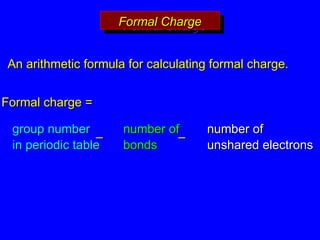
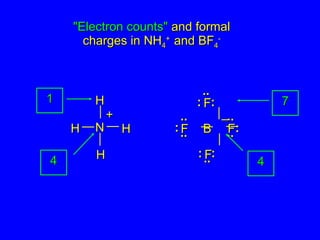
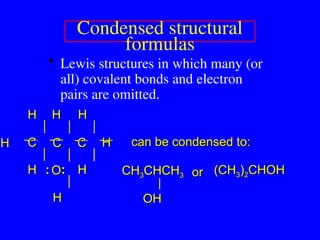
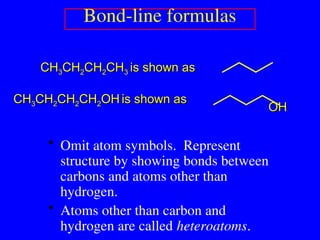
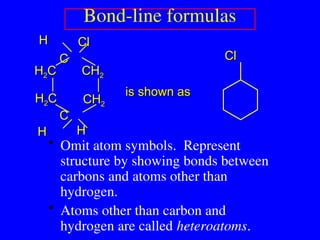
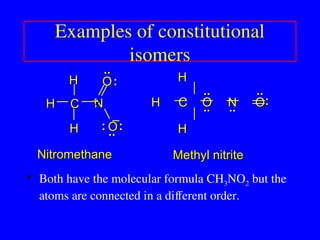
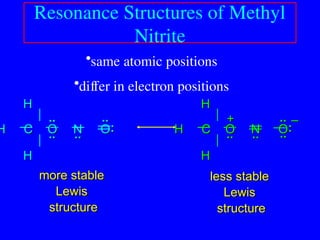
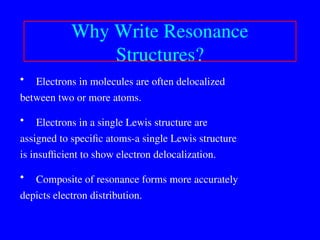
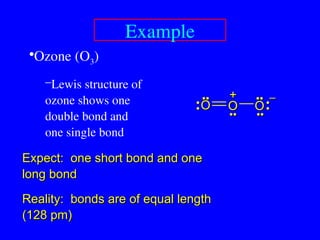
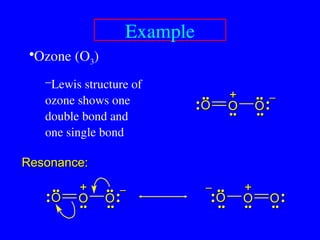
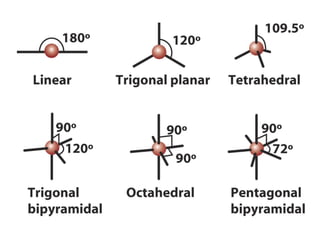
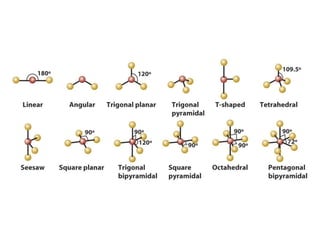
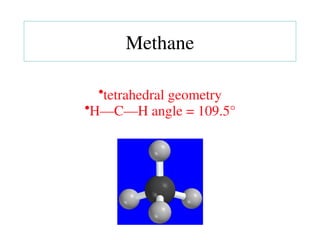
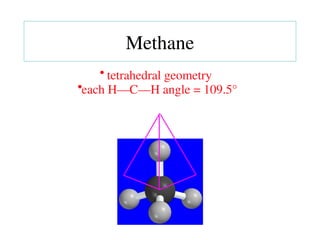
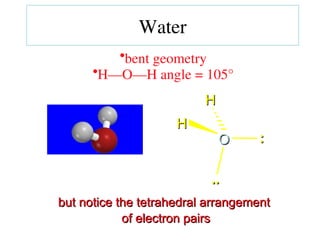
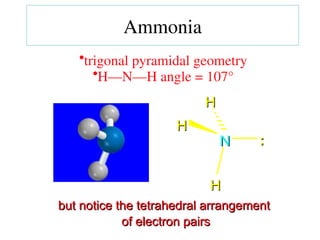
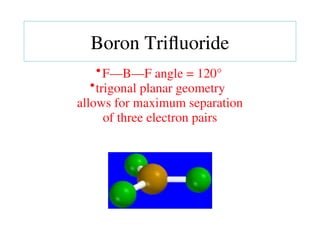
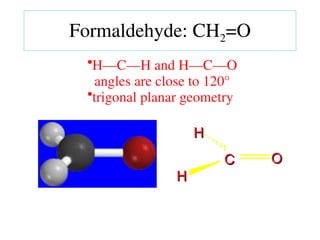
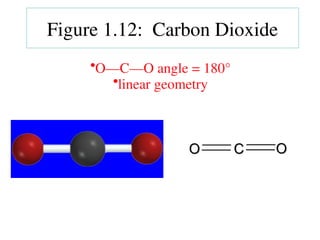
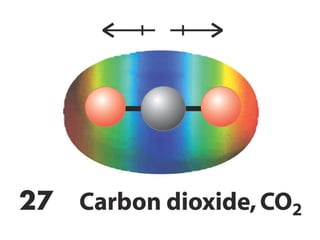
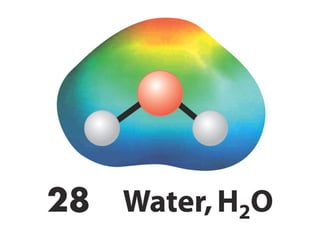
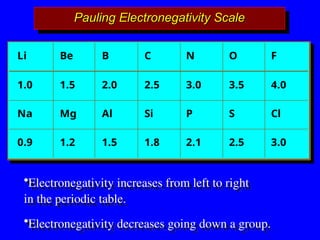
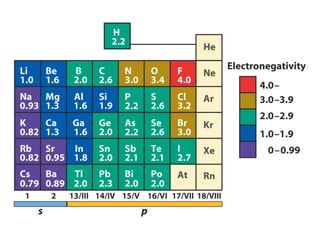
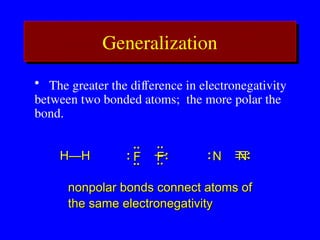
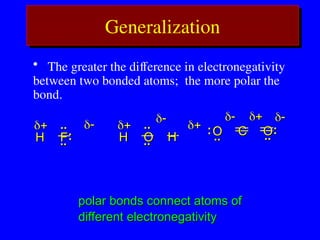
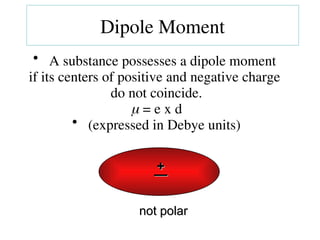
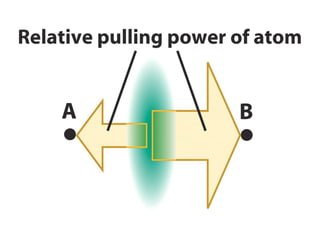
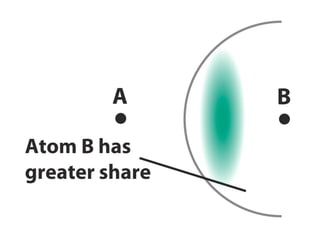
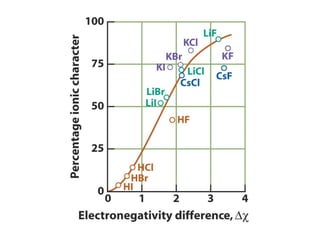
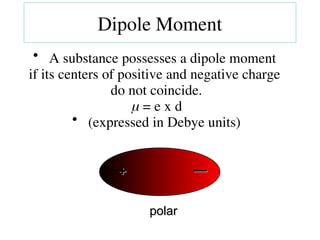
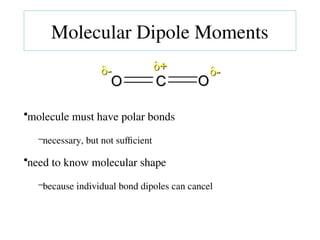
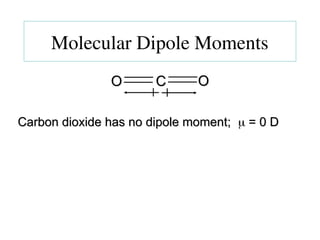
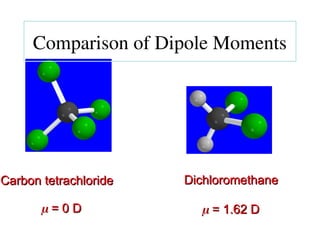
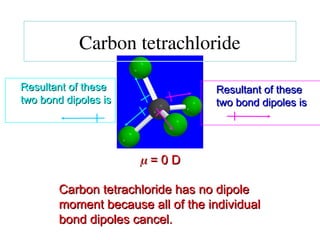
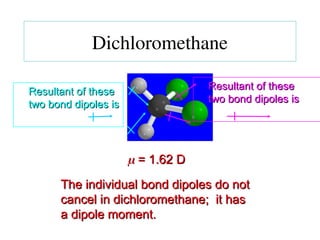
The document covers covalent bonding and Lewis structures, focusing on writing valid Lewis structures, predicting molecular geometry using VSEPR theory, and understanding electronegativity and dipole moments. It explains the principles of Lewis structures, including the octet rule, formal charges, and different types of bonds, while outlining a systematic approach to draw Lewis structures. Additionally, it discusses concepts such as constitutional isomers and resonance structures with relevant examples.