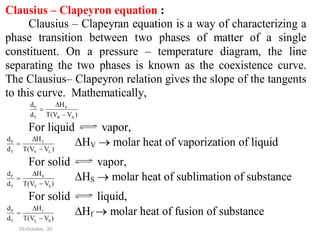
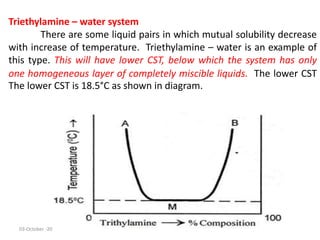
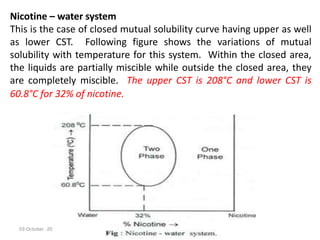
This document discusses phase transitions and the Clausius-Clapeyron equation. It defines phase transition as the transformation of a thermodynamic system from one phase to another with uniform physical properties. The Clausius-Clapeyron equation characterizes phase transitions between two phases and relates the slope of the coexistence curve to changes in enthalpy, temperature, and volume. Finally, it describes partially miscible liquid systems like phenol-water, triethylamine-water, and nicotine-water and how their miscibility varies with temperature and composition.