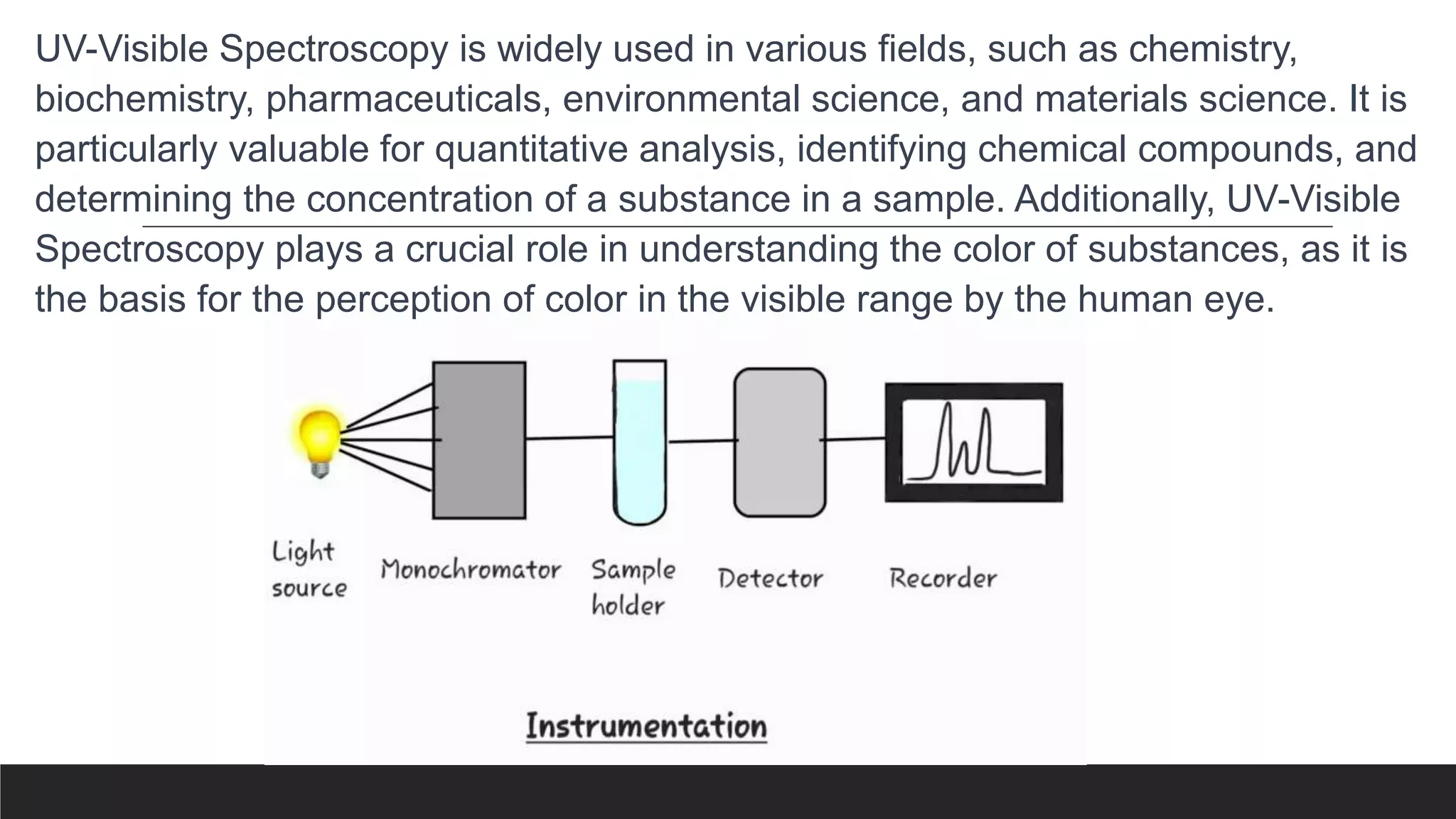
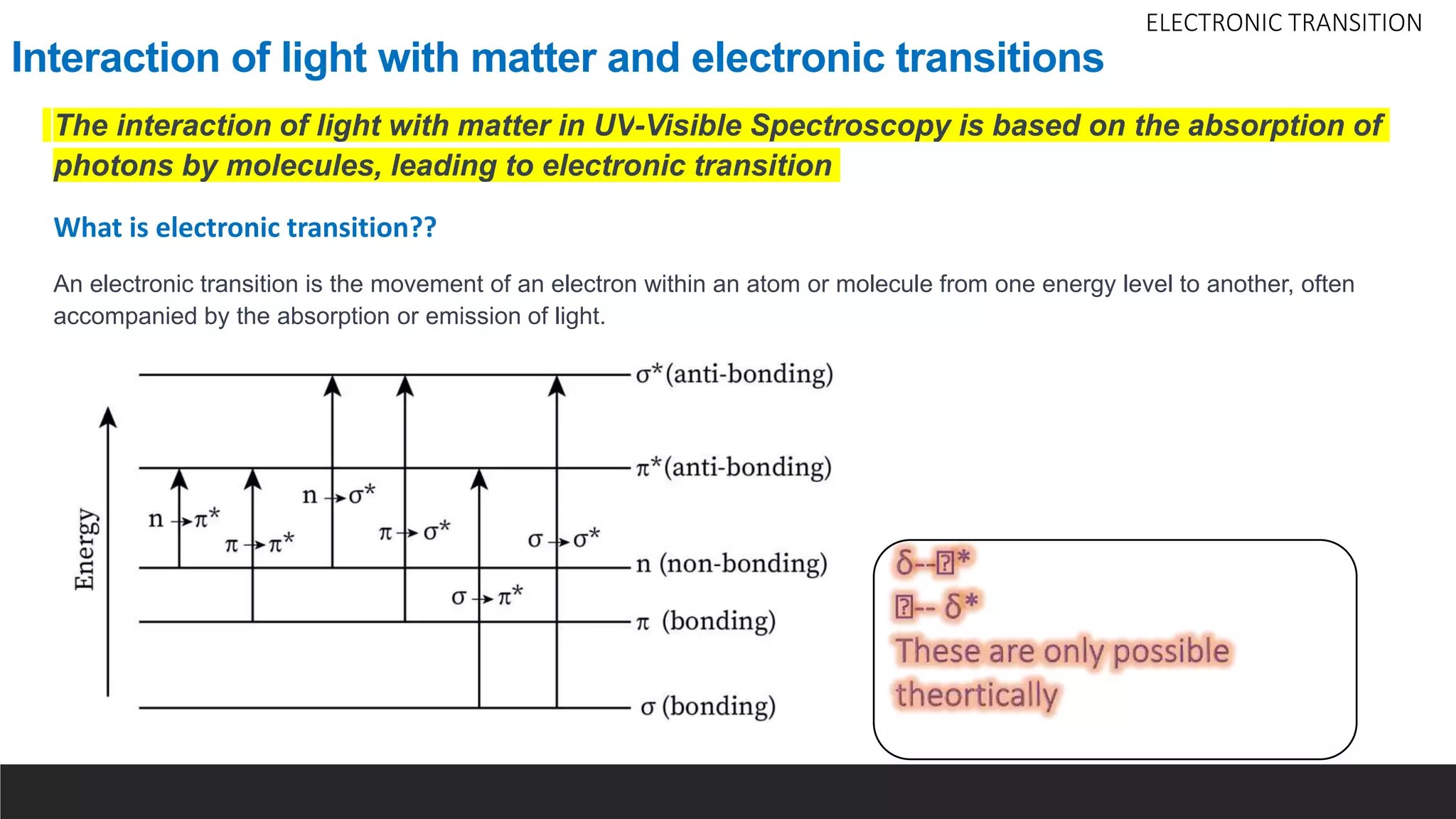
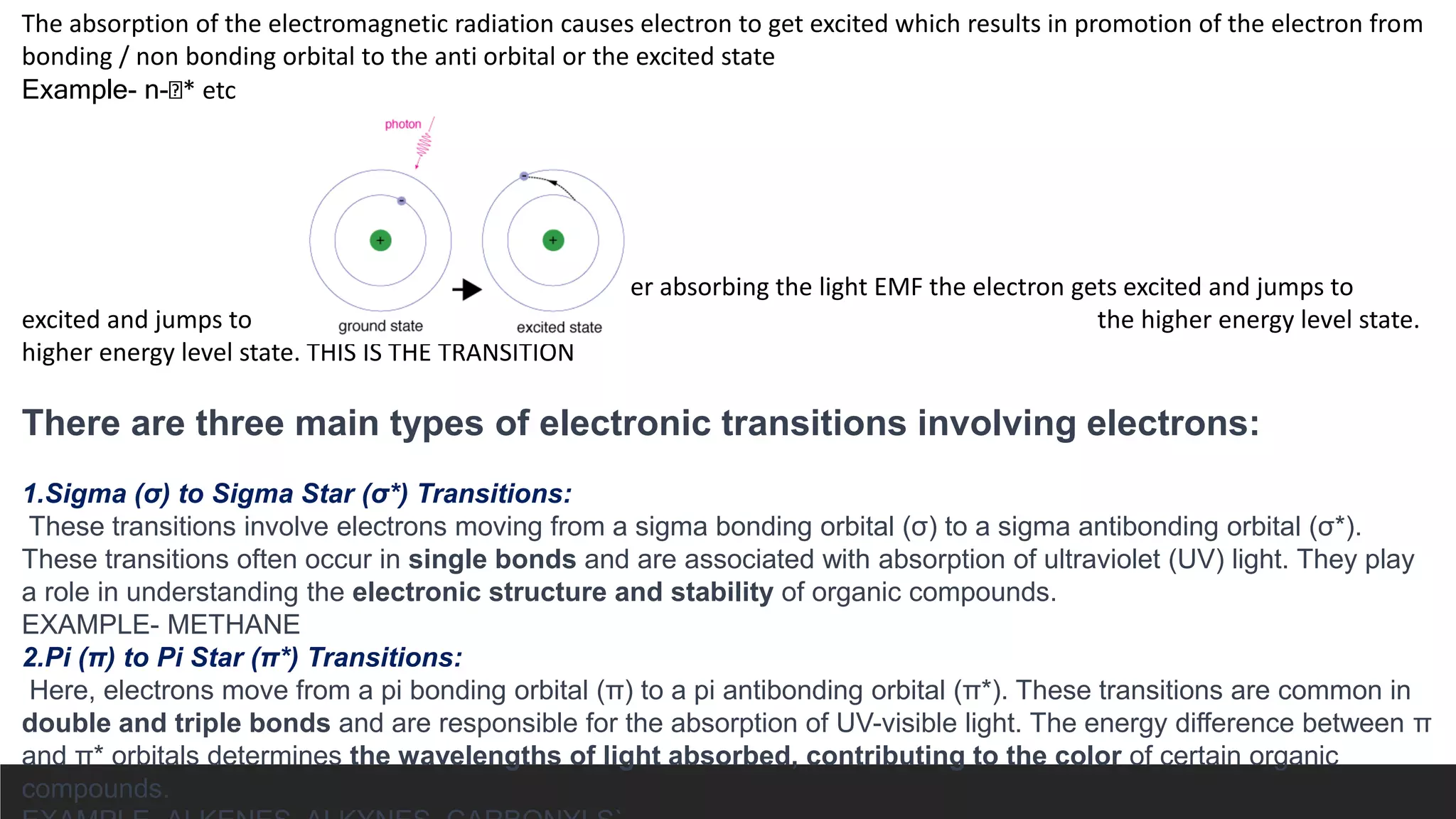
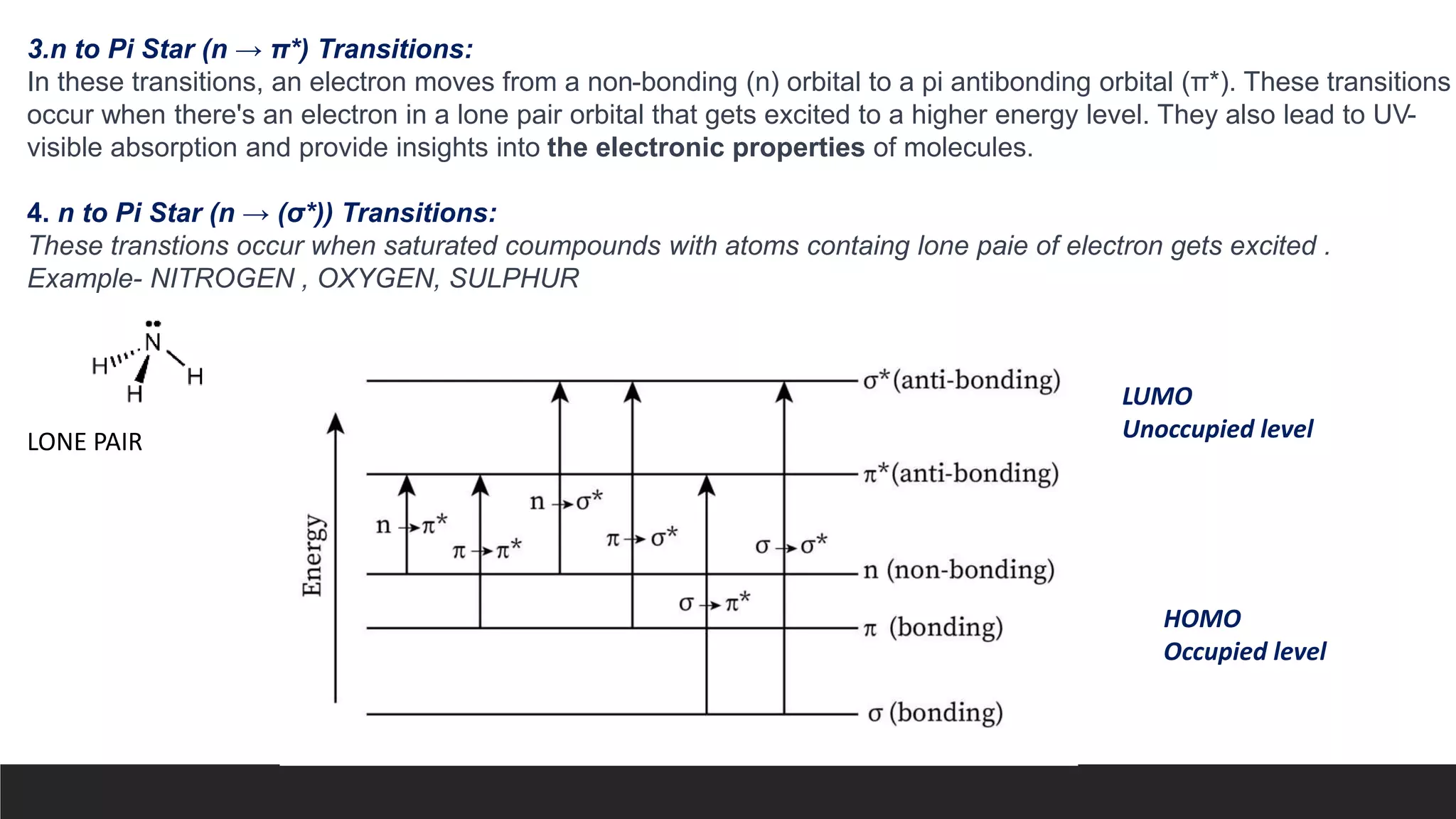
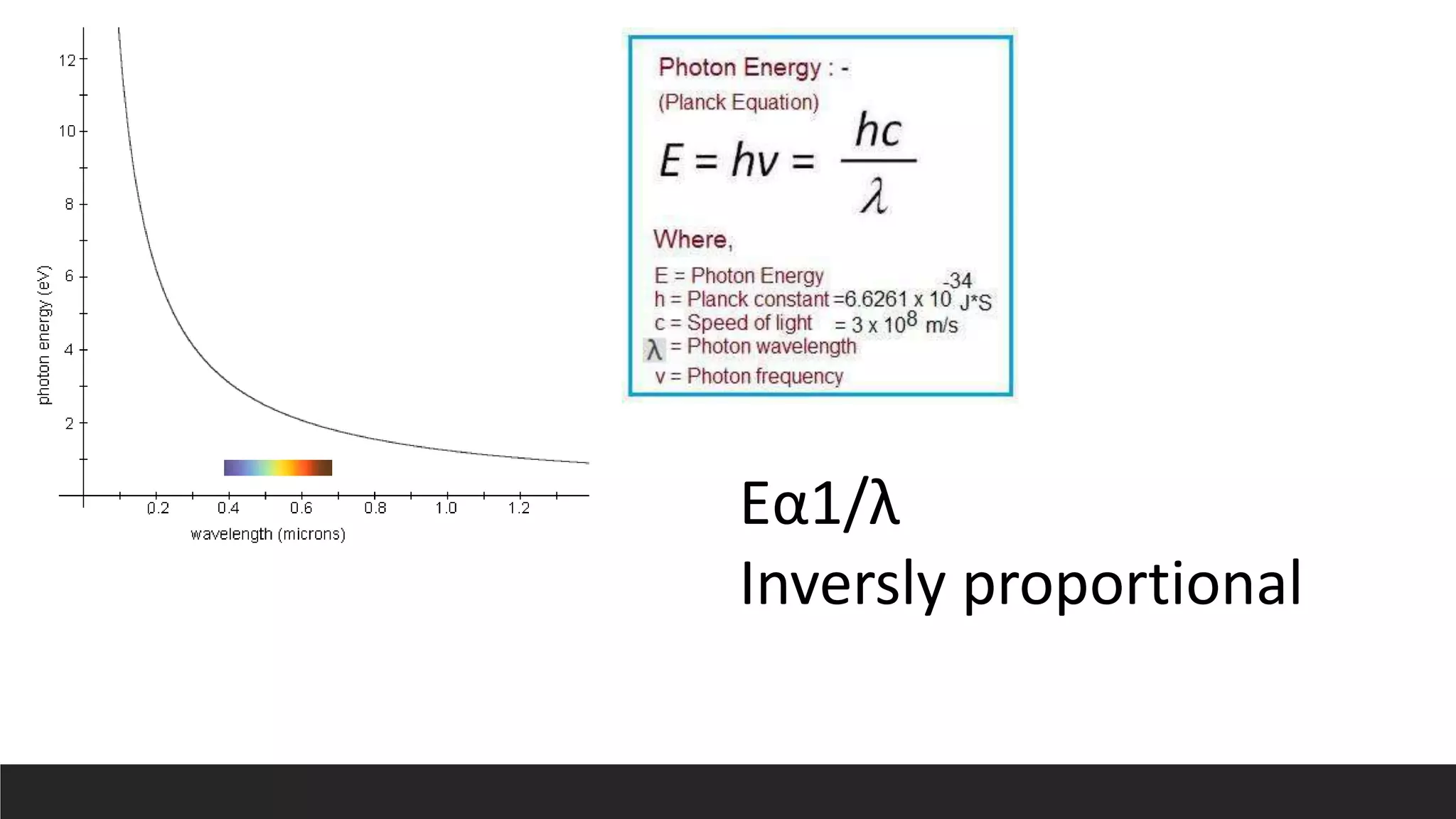
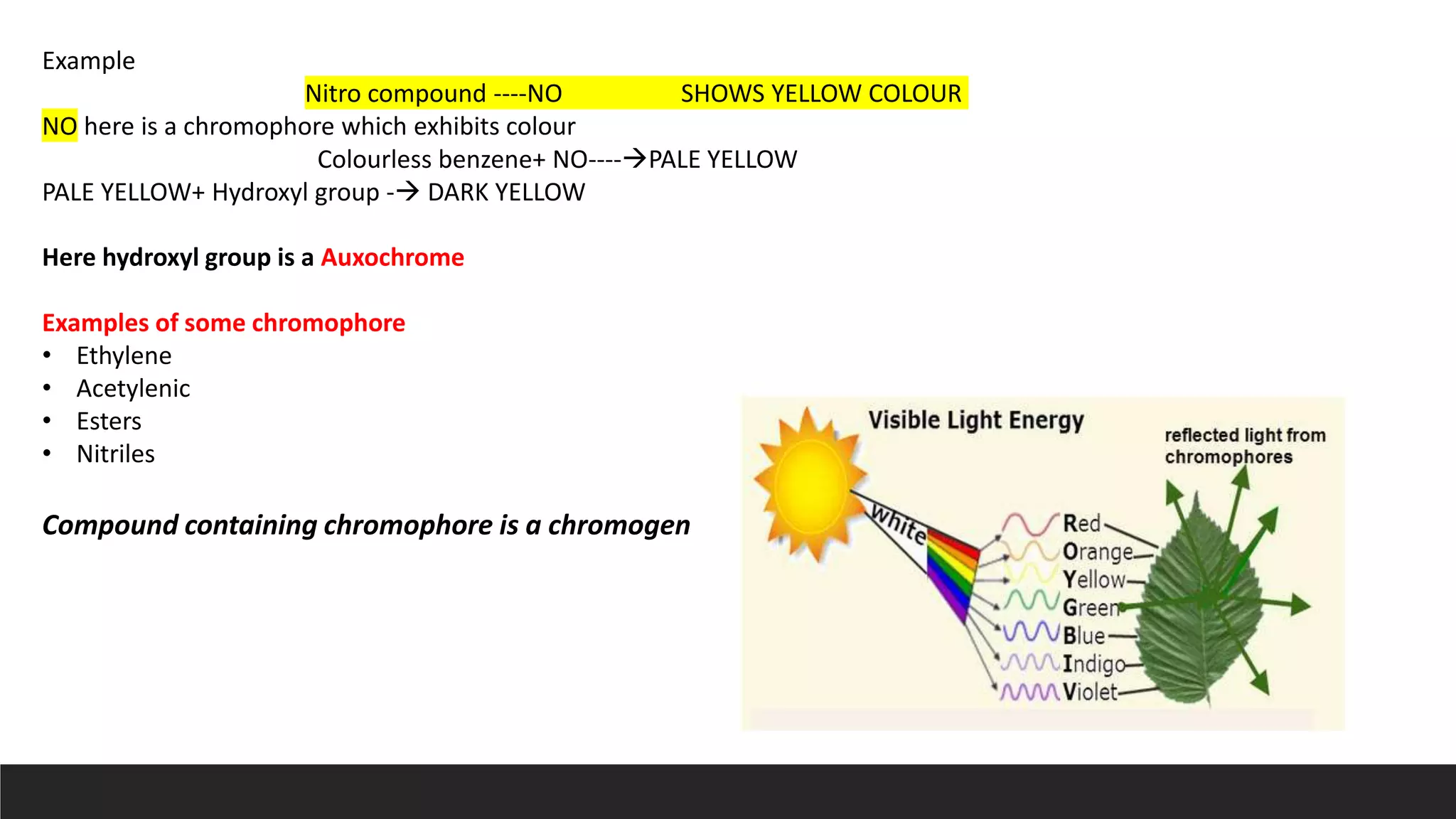
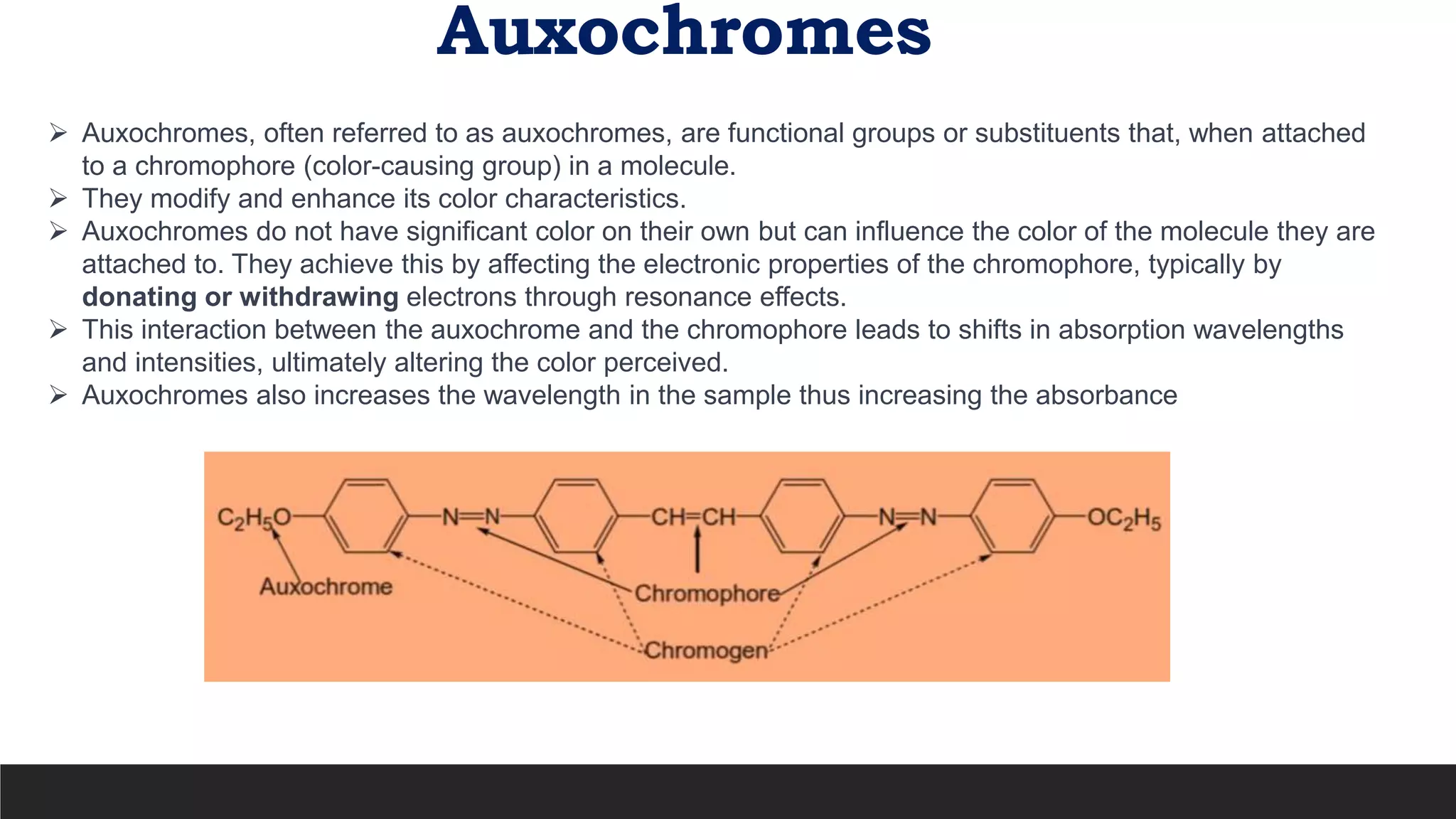
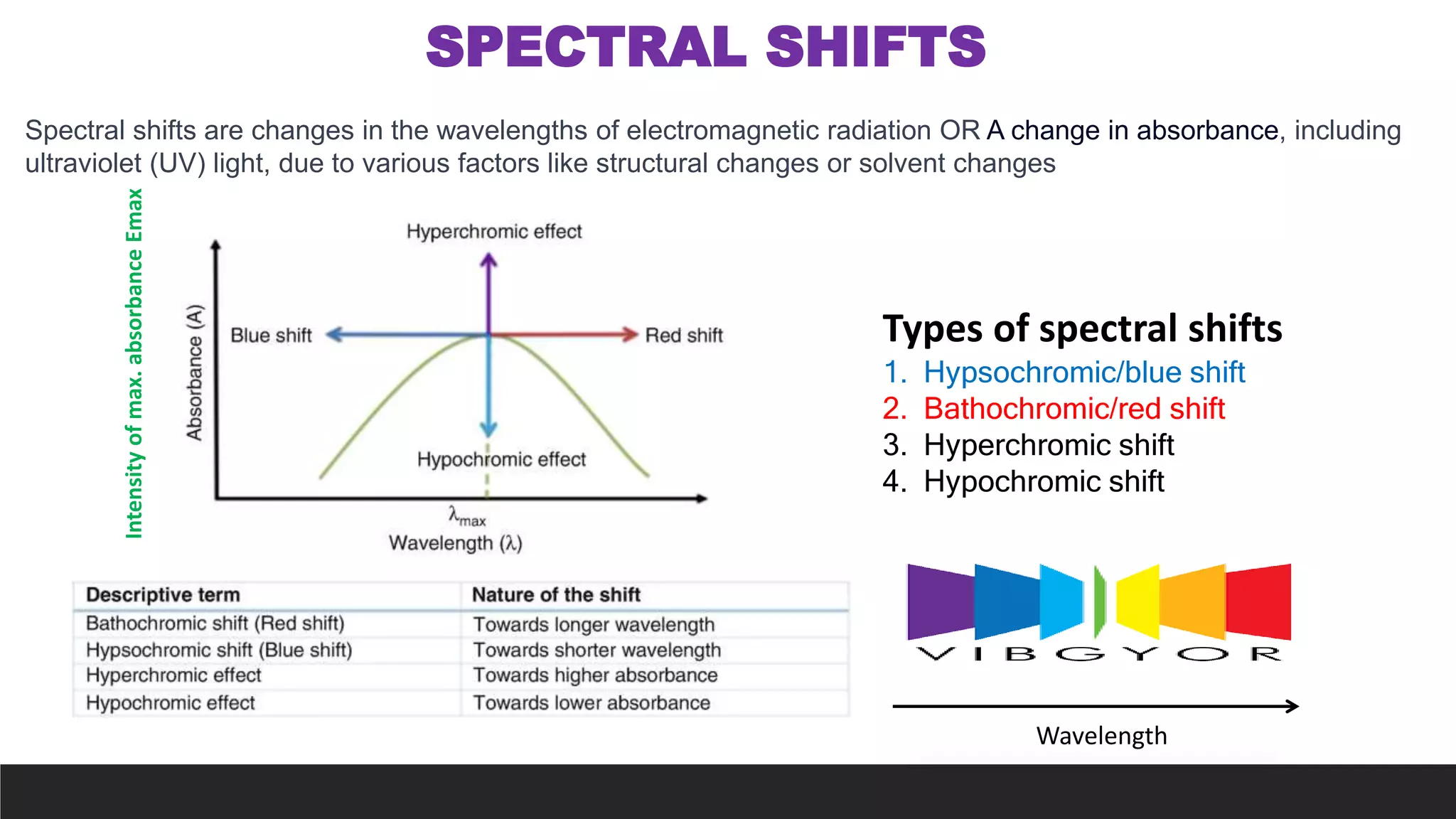
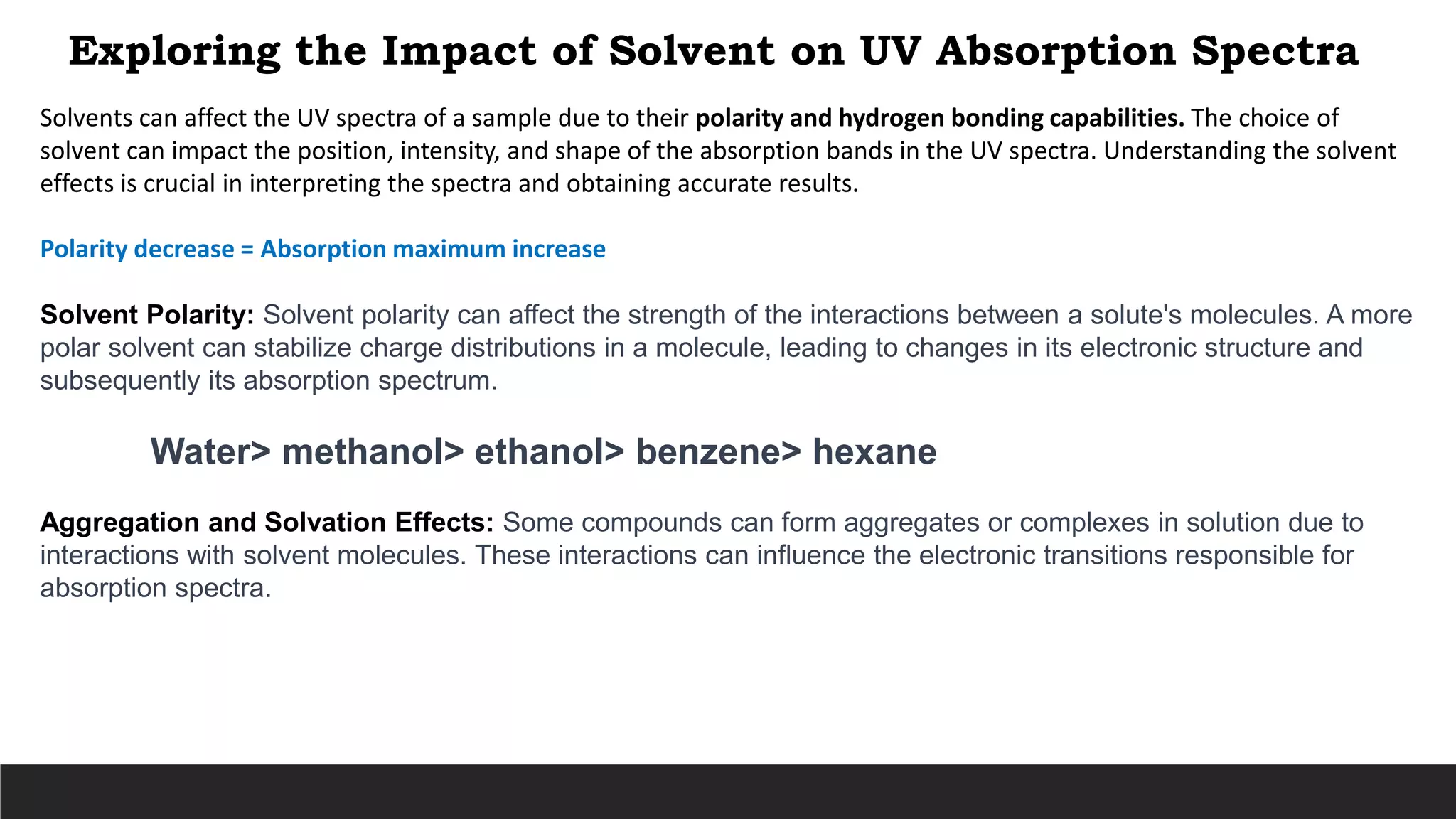
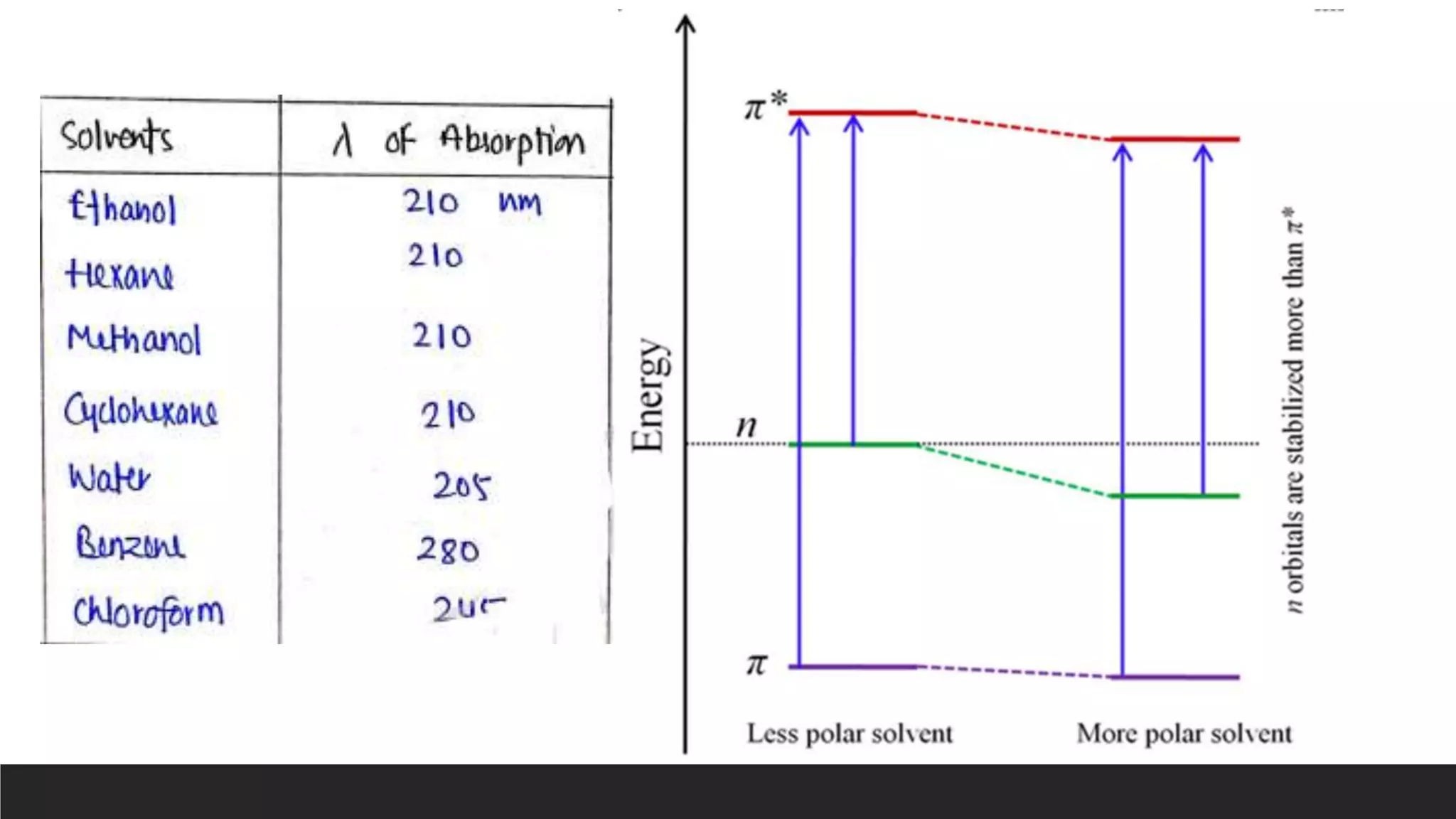
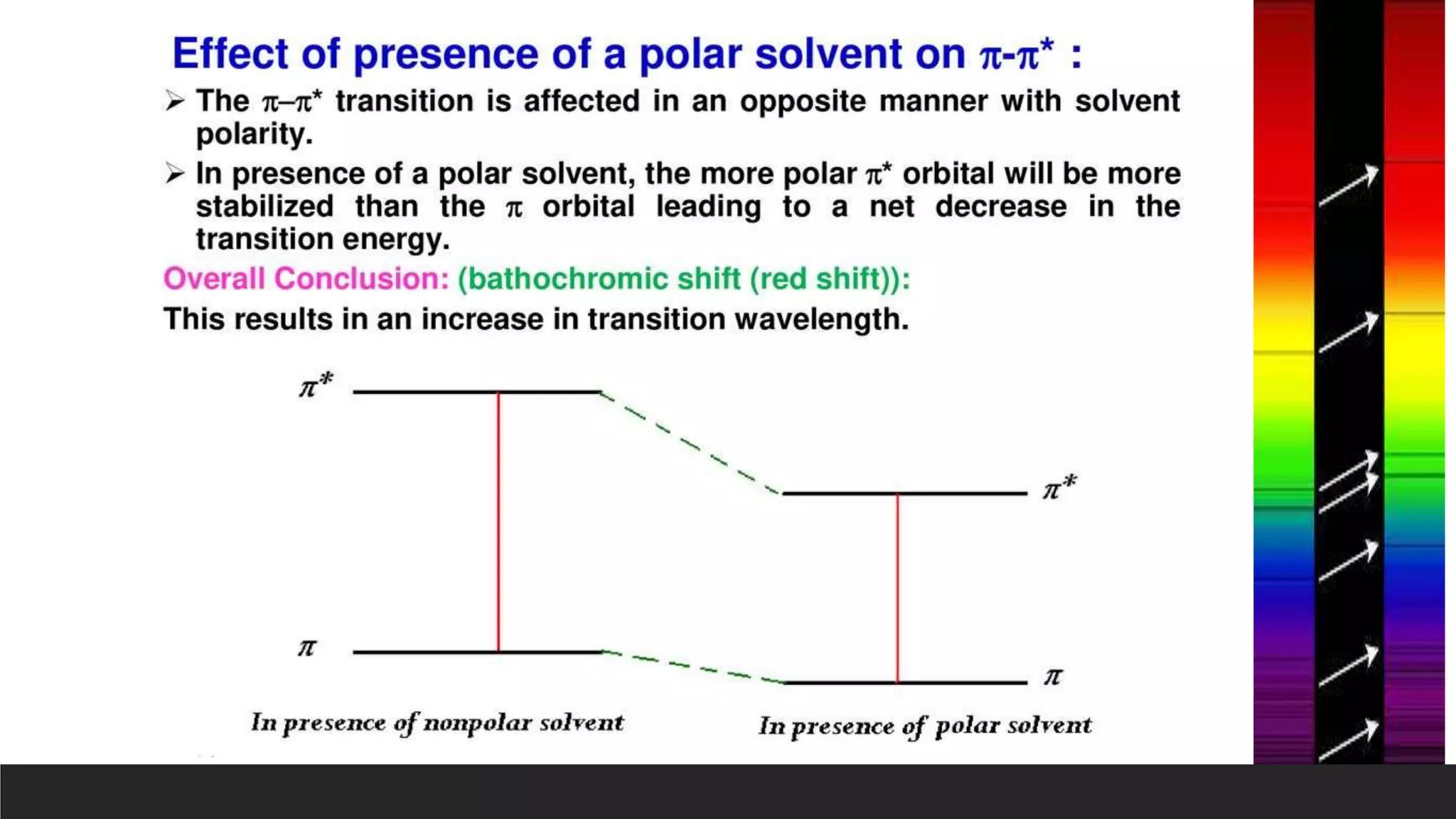
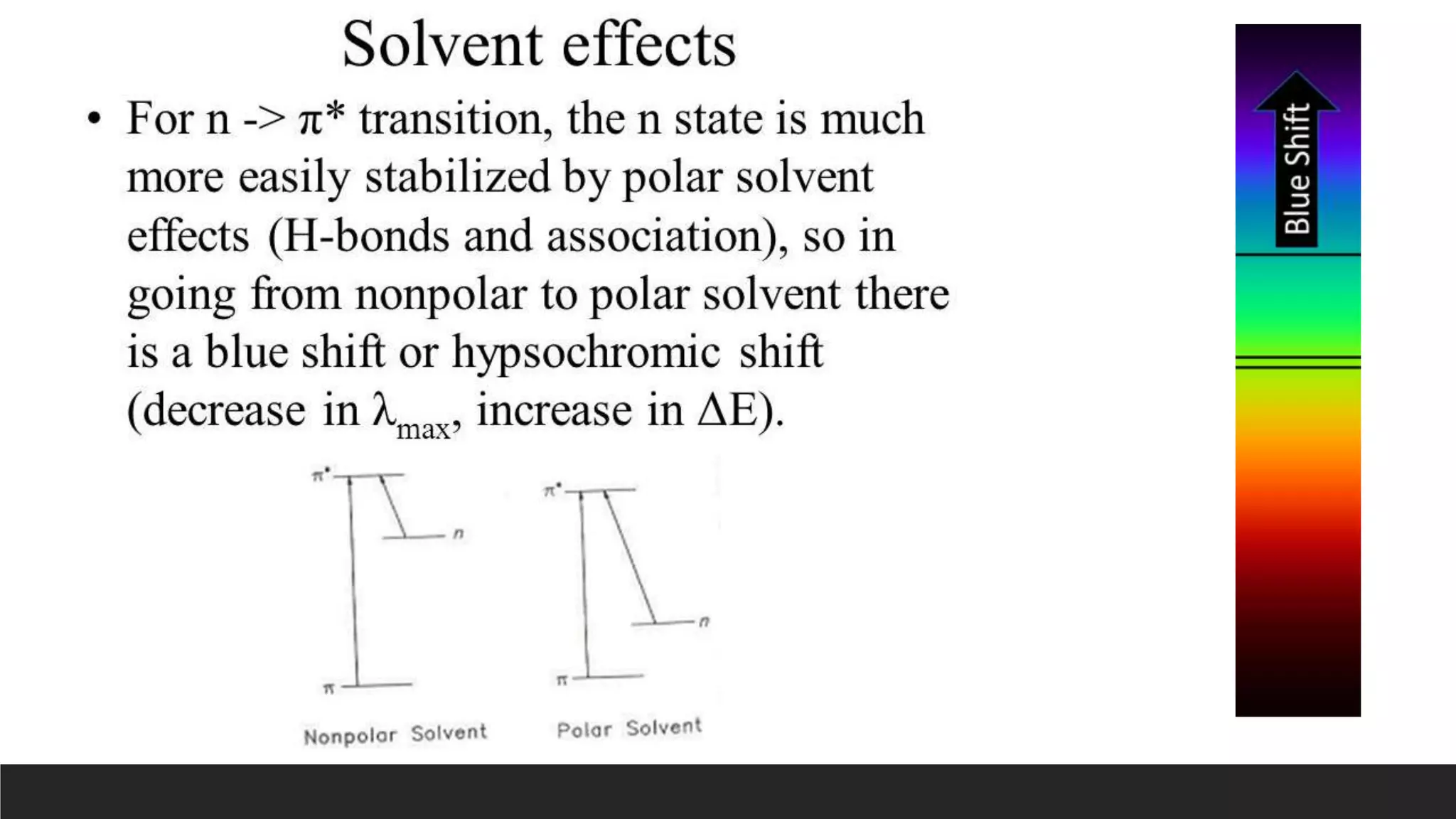
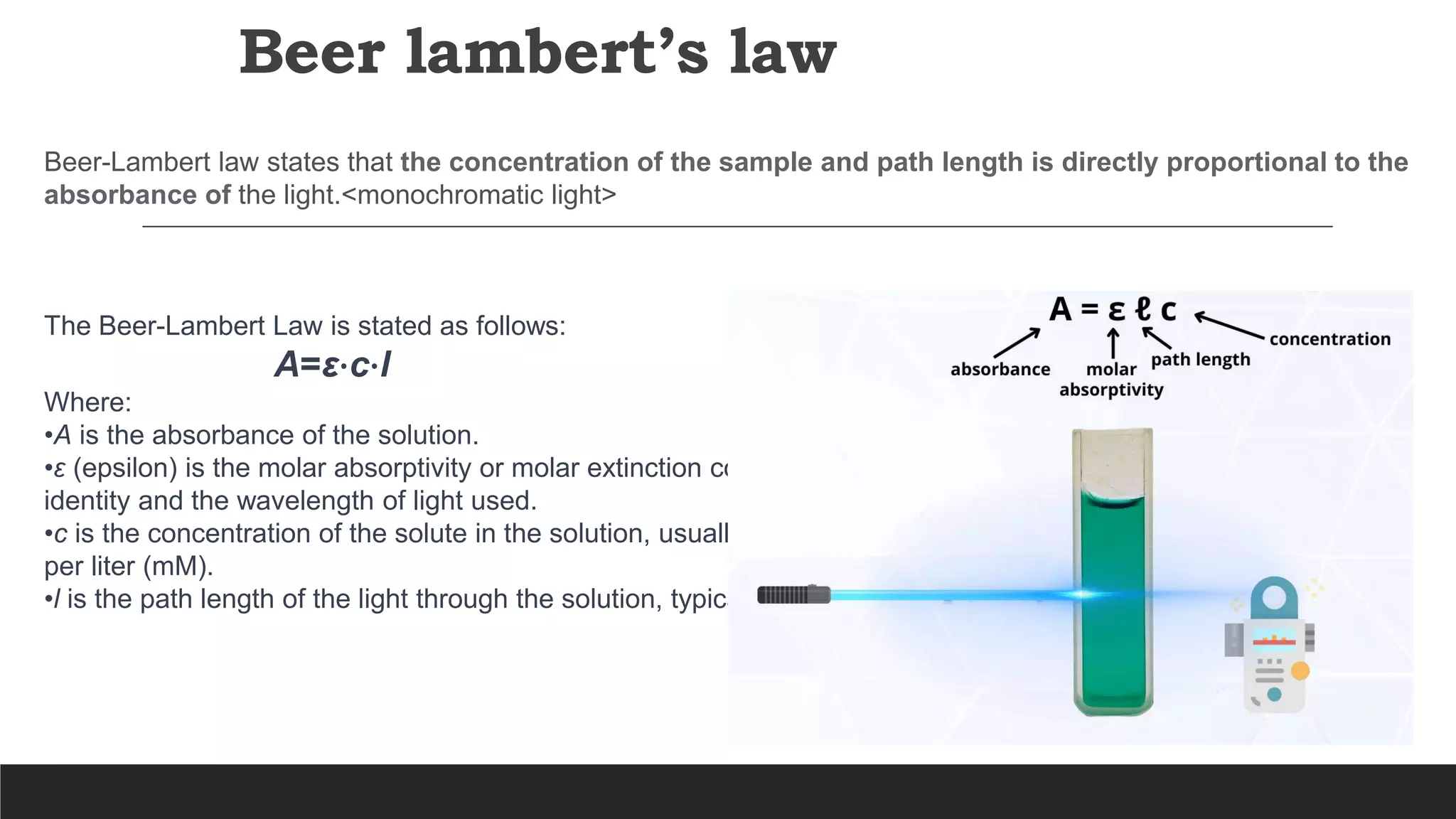
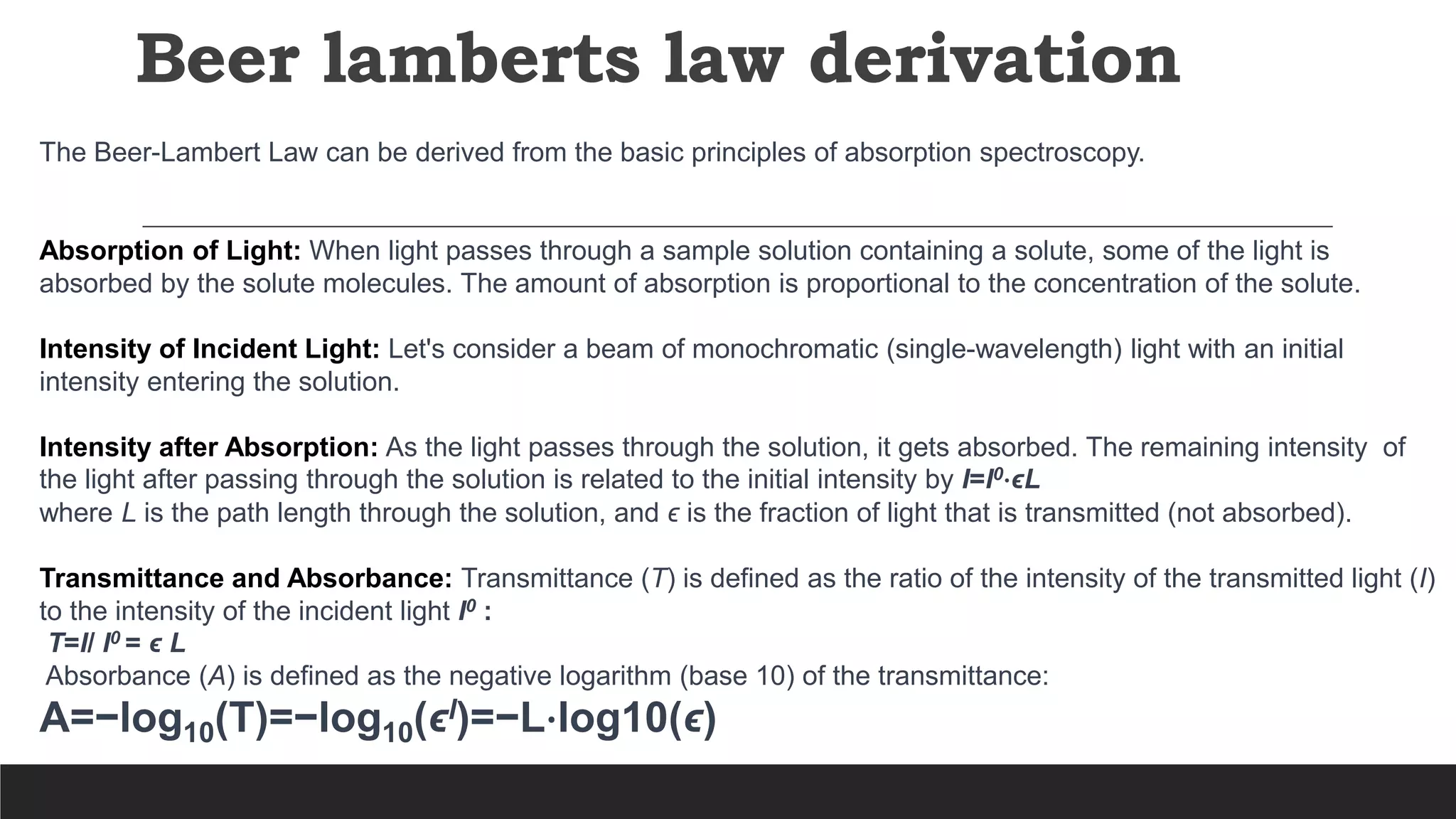
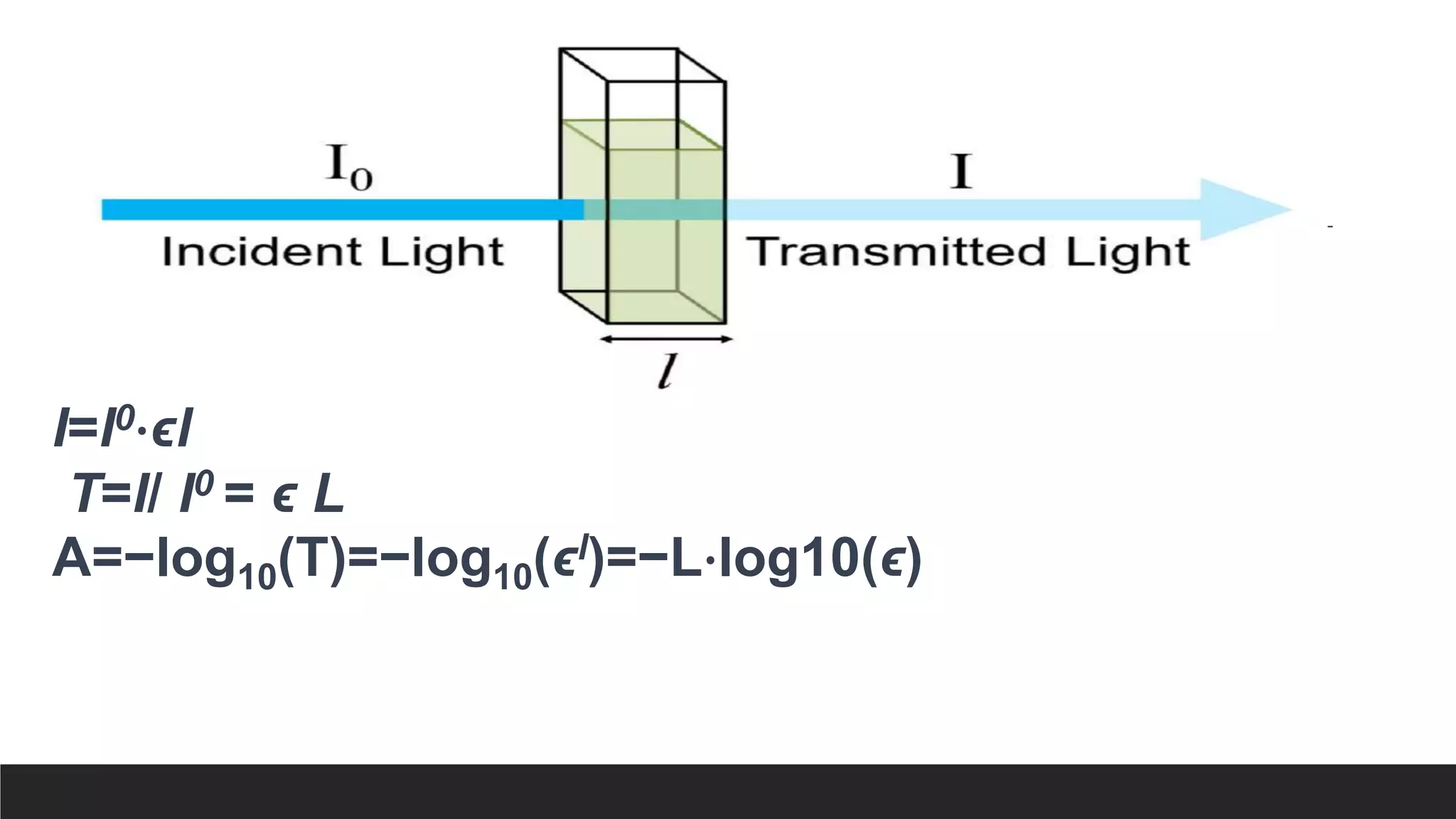
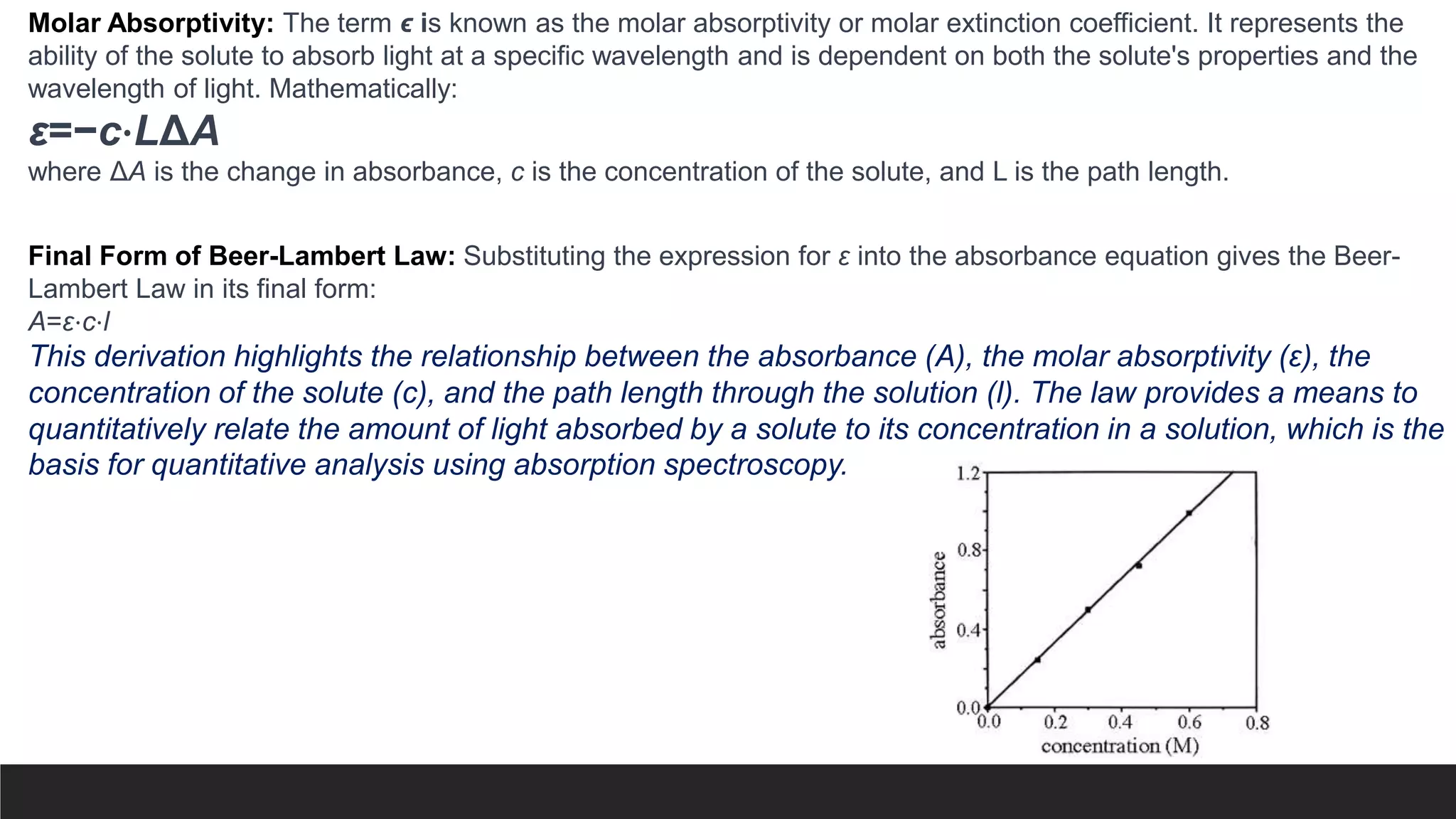
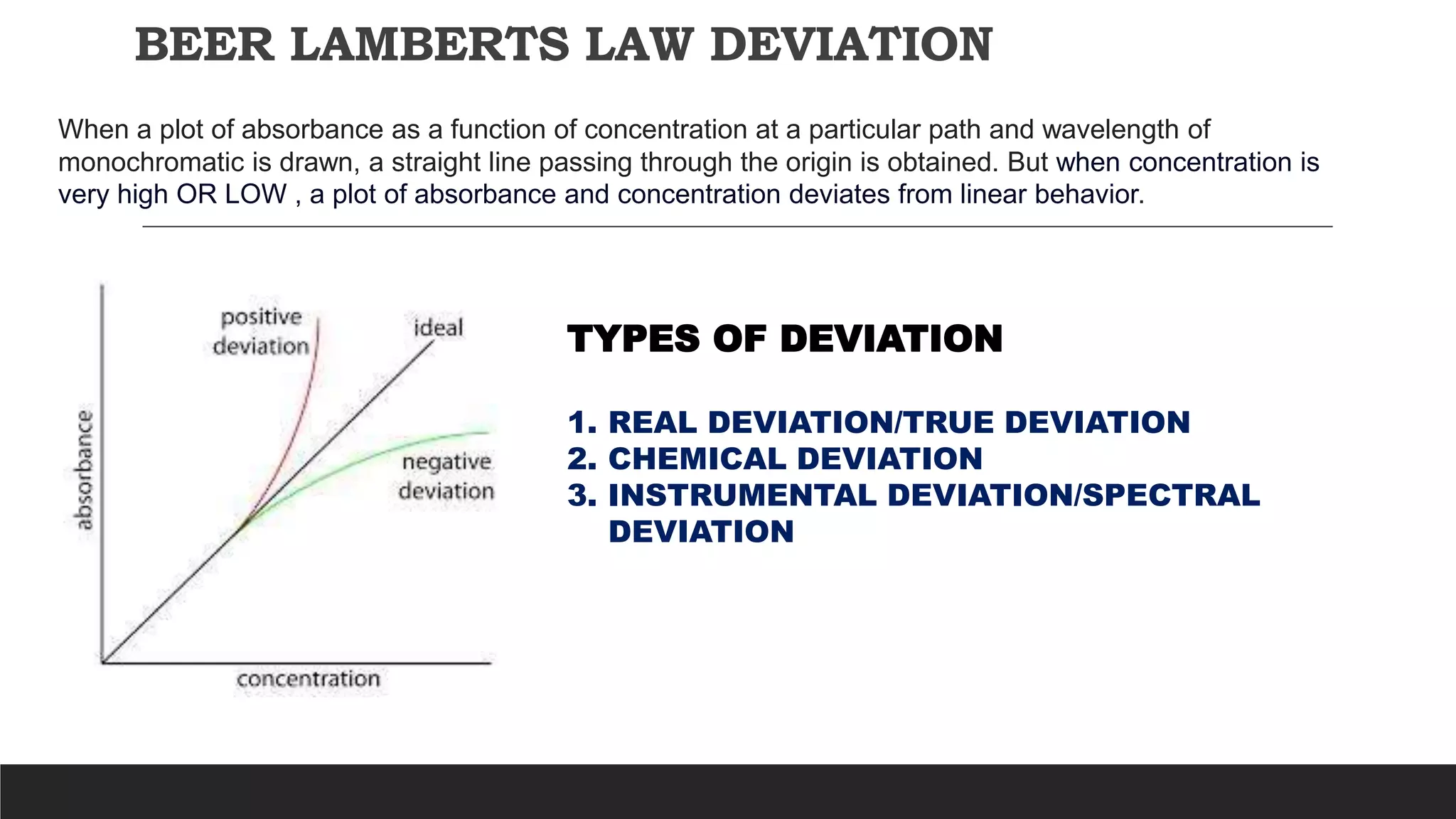
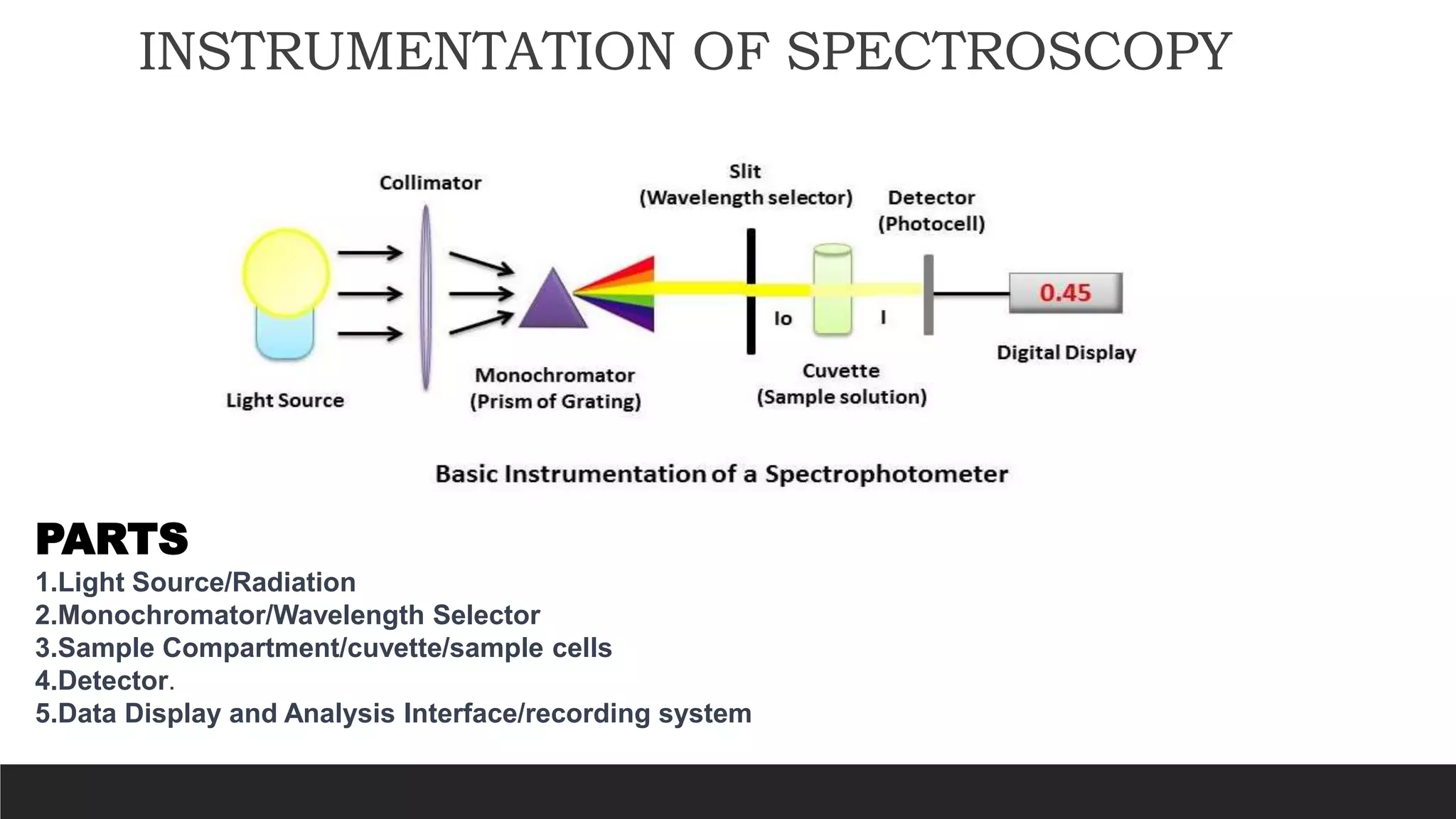
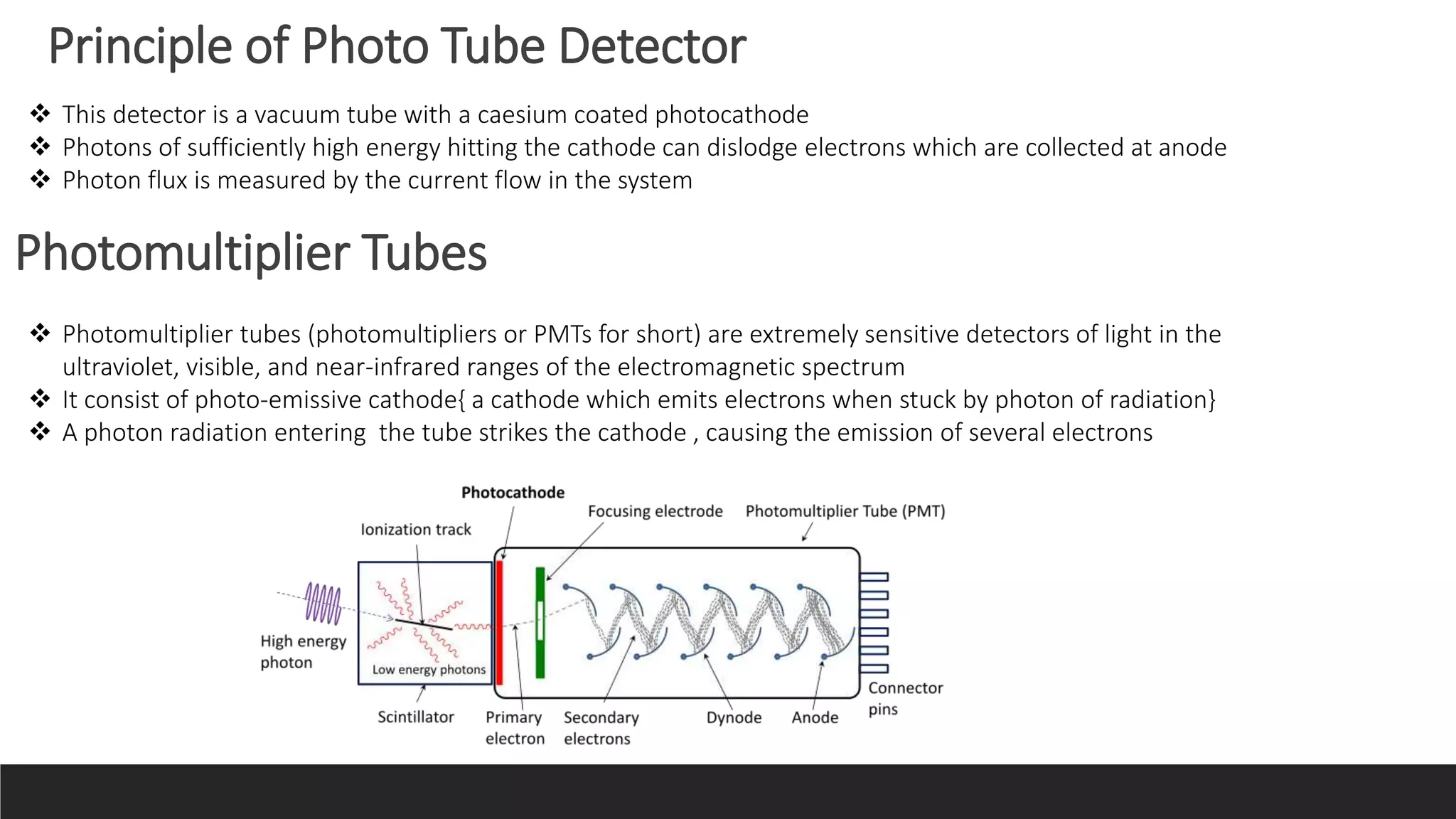
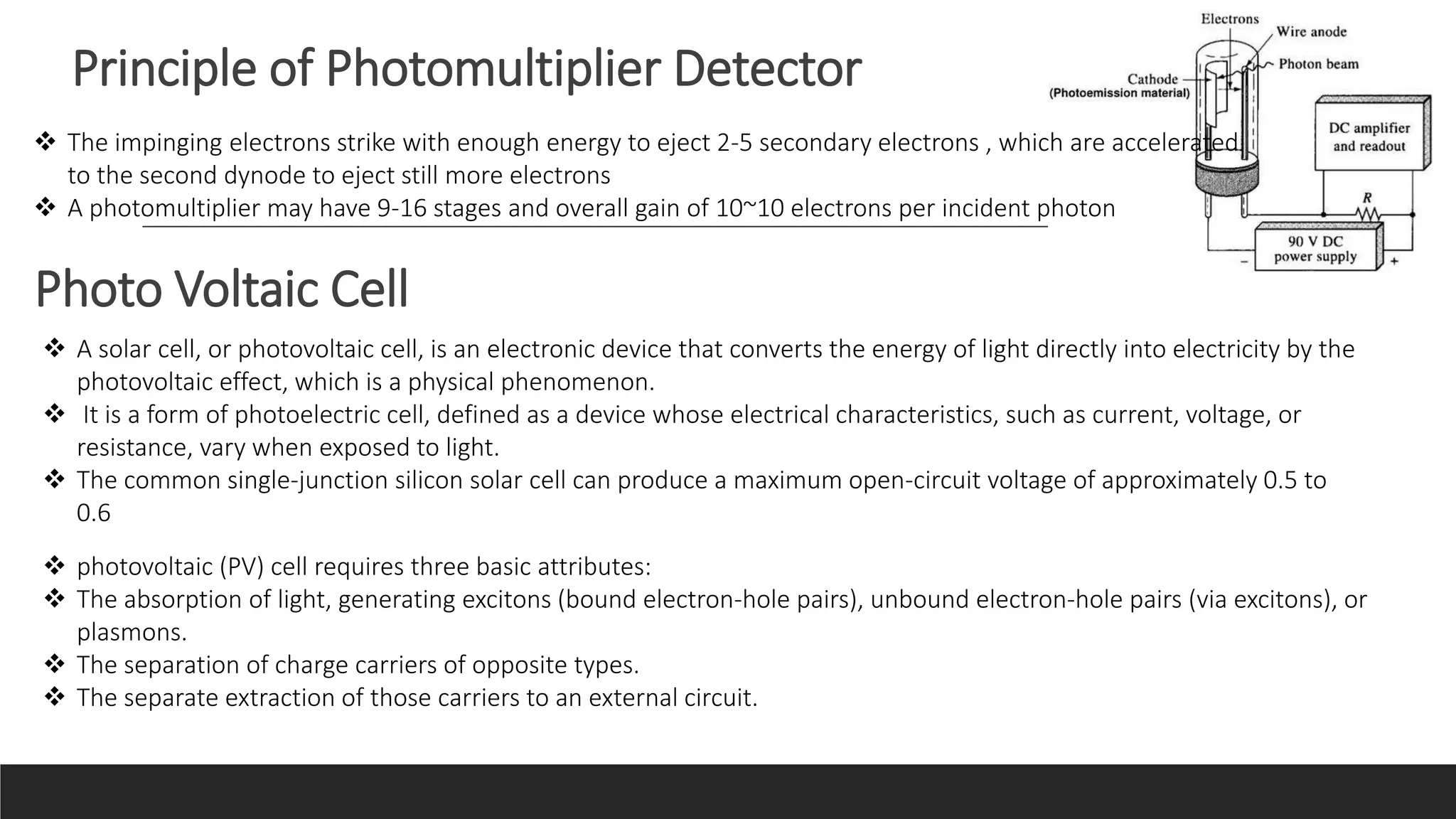
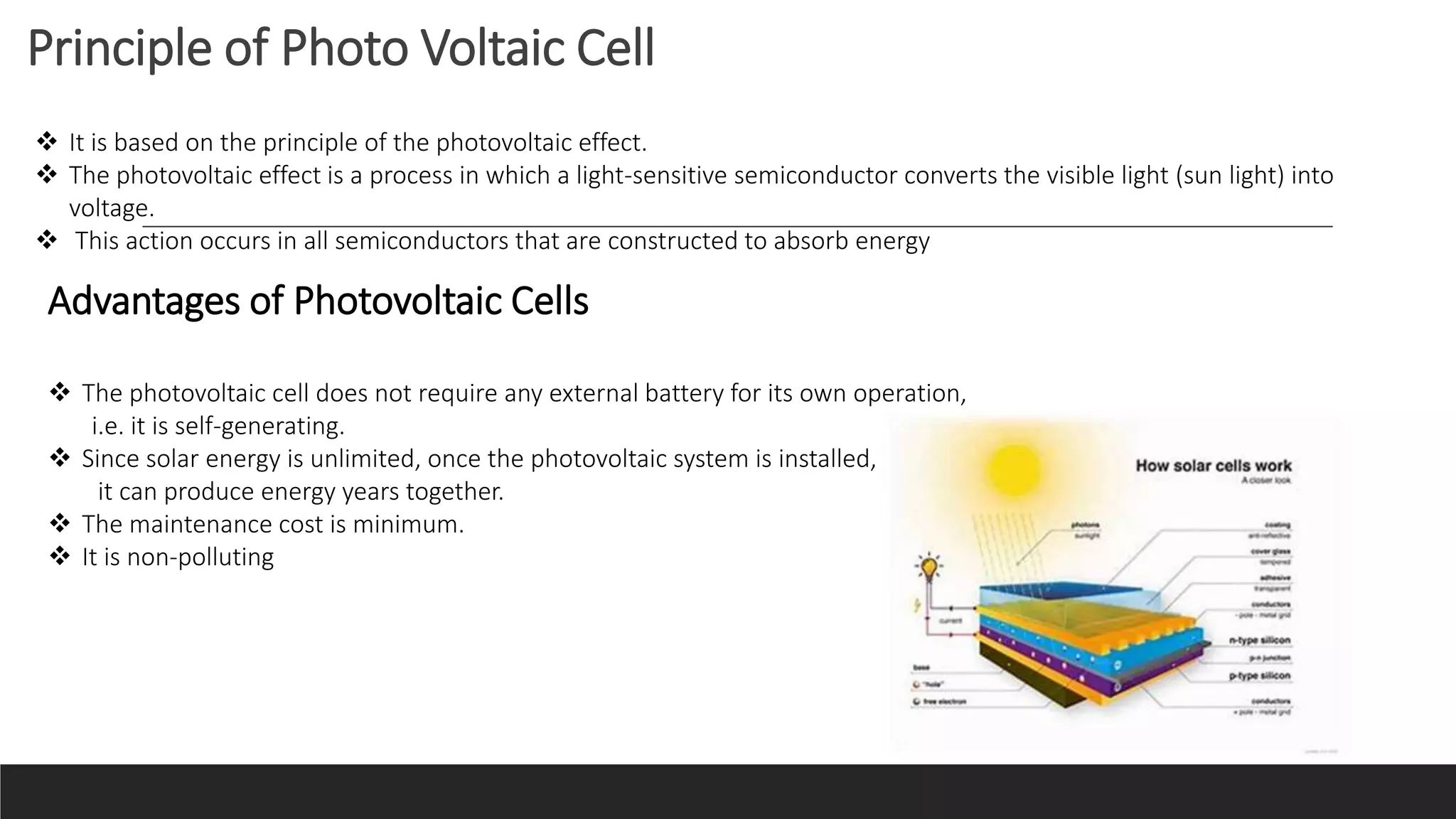
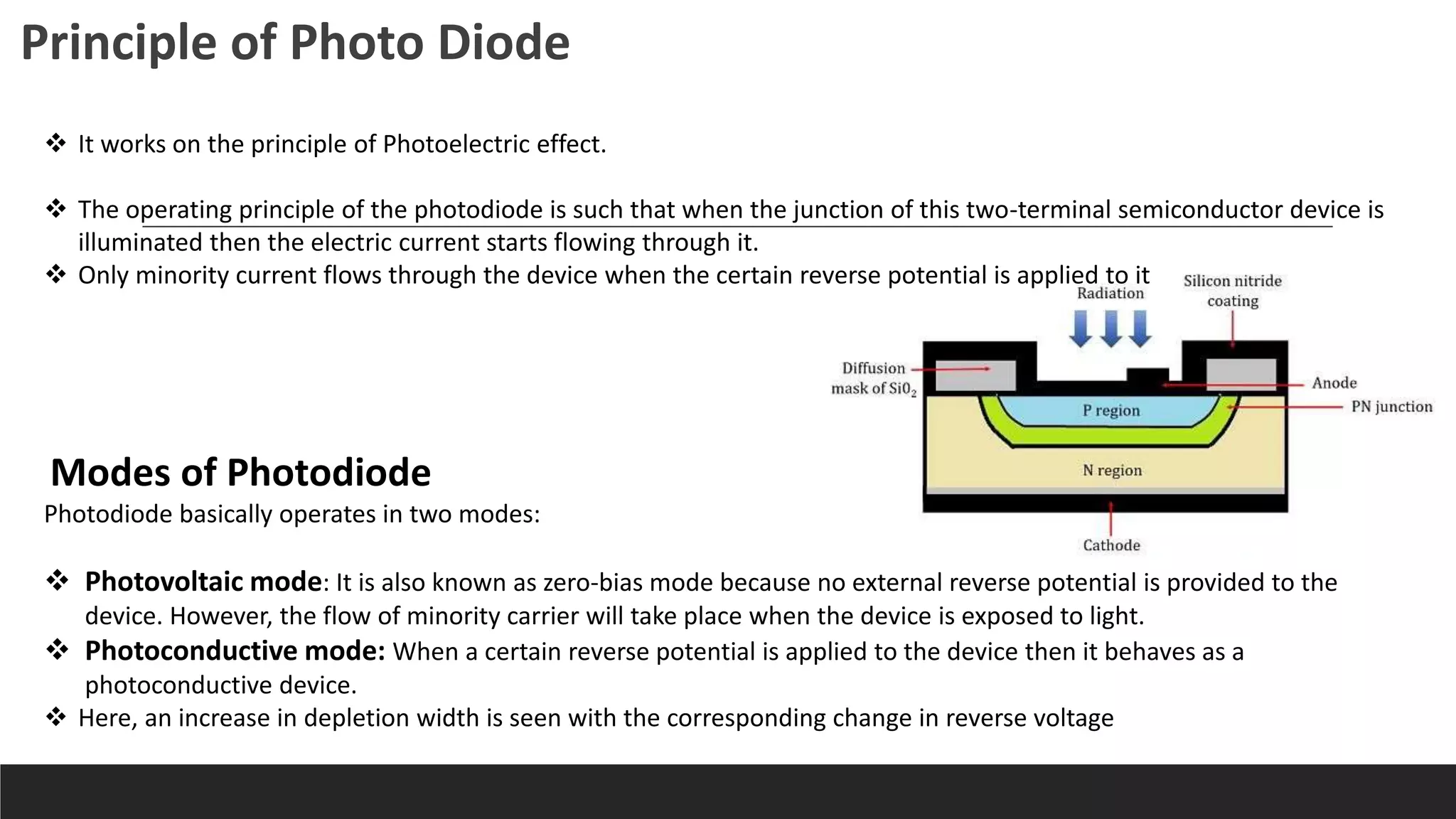
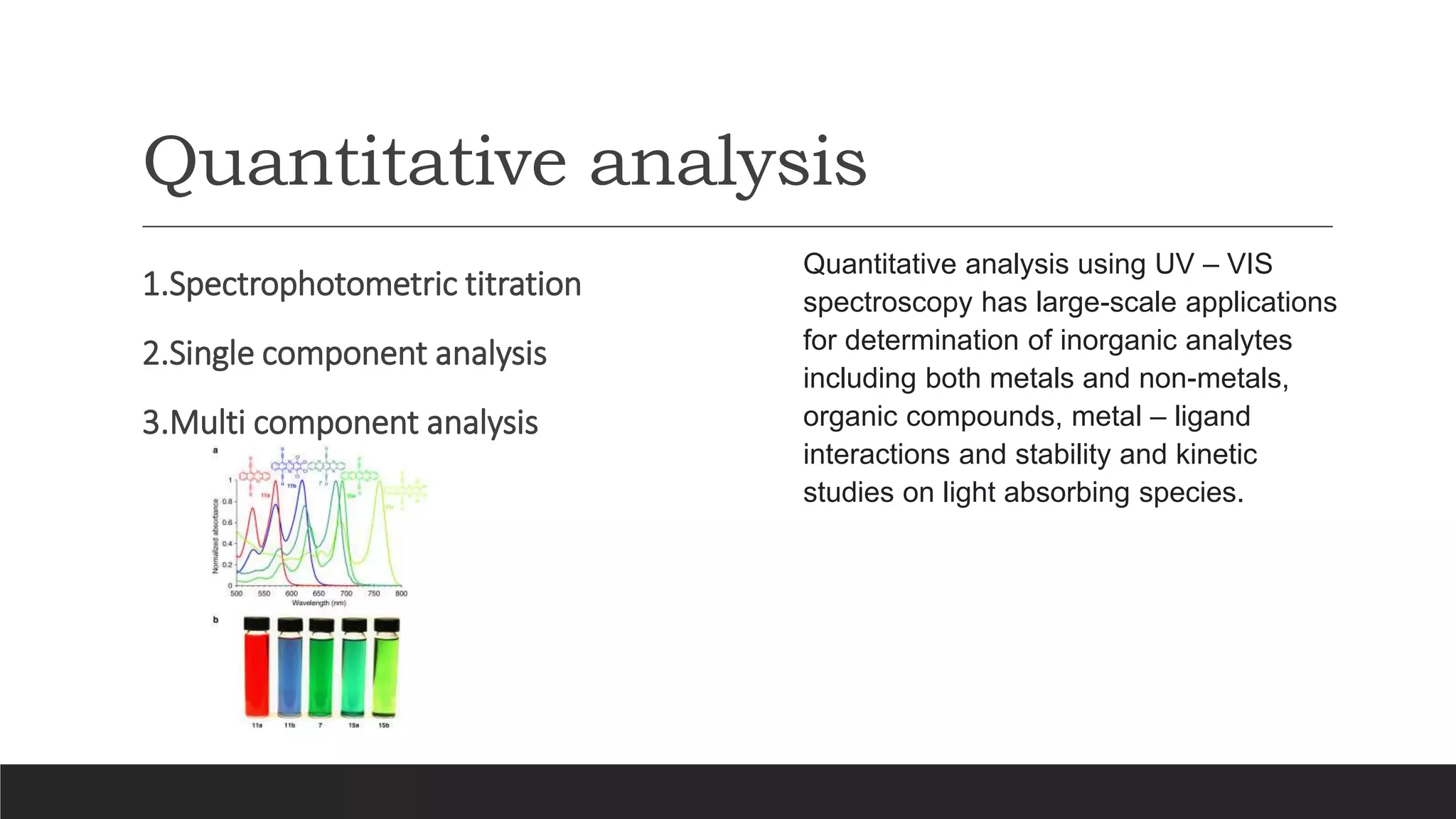
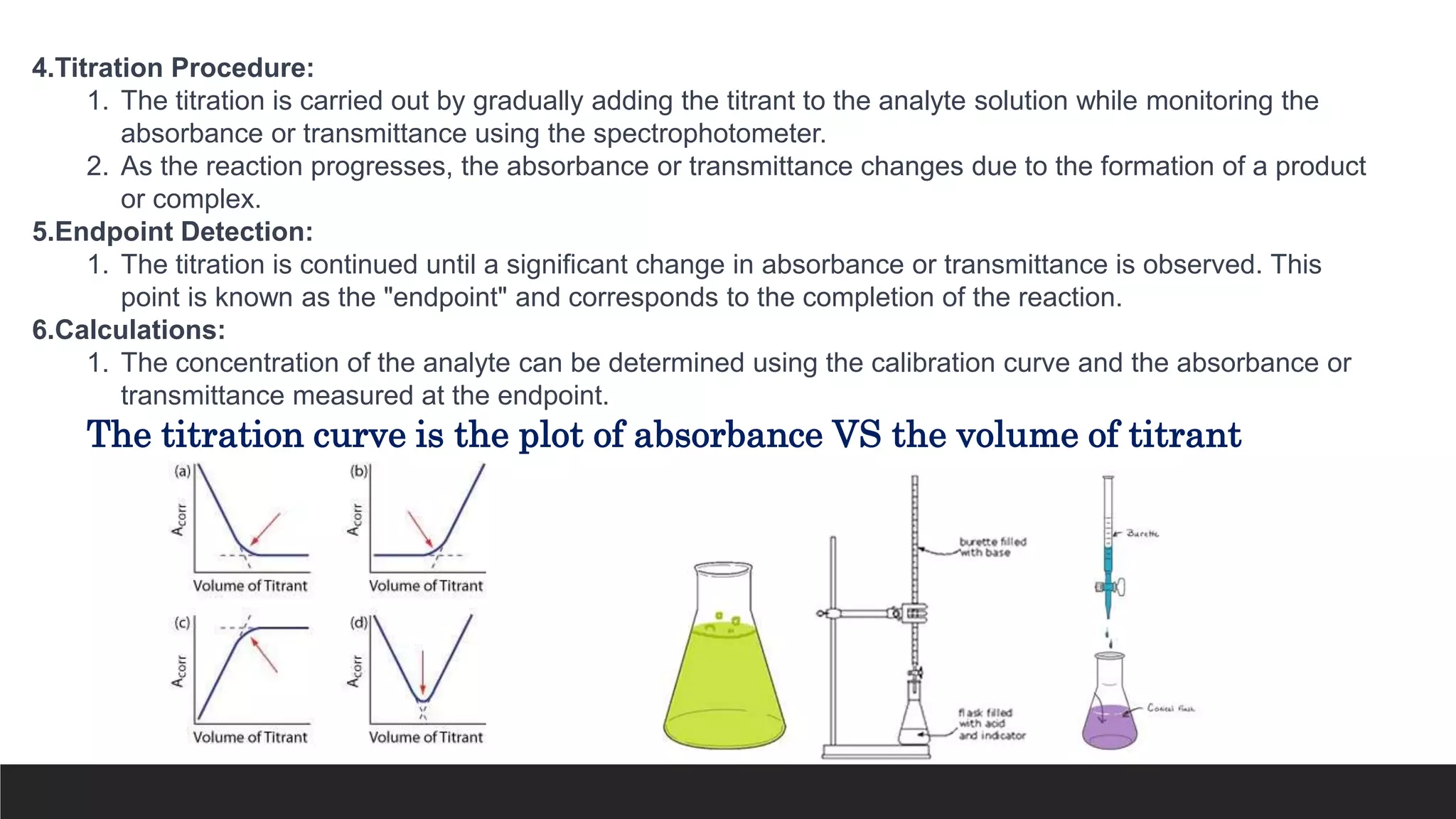
UV-Visible Spectroscopy is a technique that uses light in the UV and visible range to analyze molecules. It works by measuring how much light a sample absorbs or transmits at different wavelengths. Electronic transitions within molecules correspond to specific absorbed wavelengths. A spectrophotometer generates light that passes through a sample, and the amount of light absorbed provides information about the sample's structure and composition. The Beer-Lambert law quantitatively relates absorbance to concentration and path length. UV-Vis spectroscopy has many applications in fields like chemistry, biochemistry, pharmaceuticals and more.