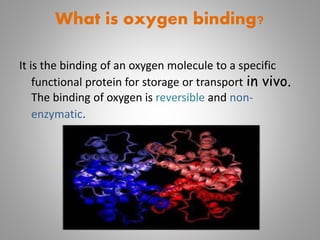
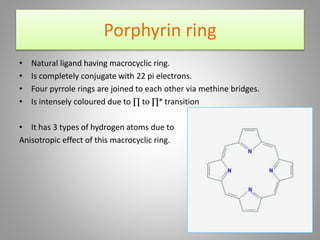
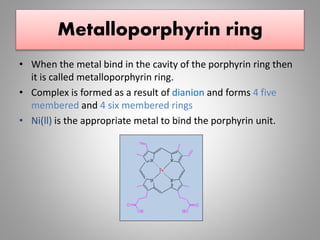
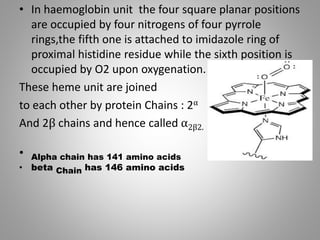
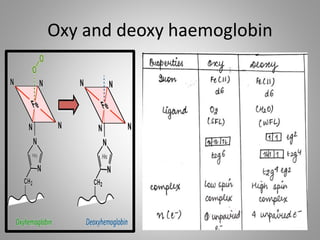
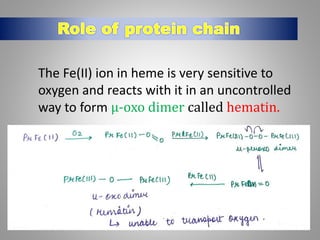
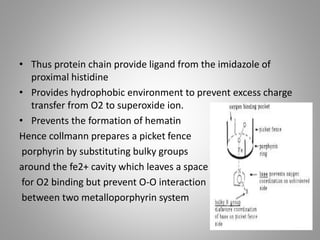
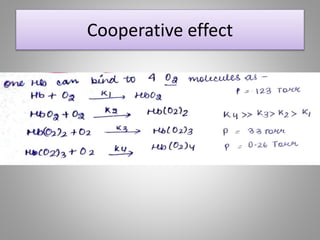
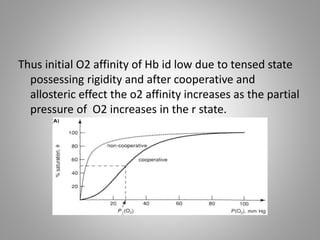
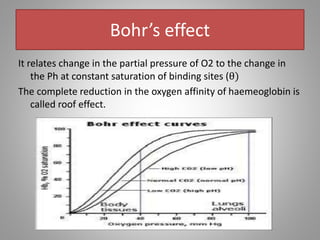
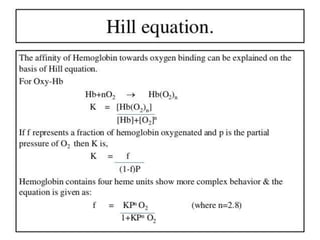
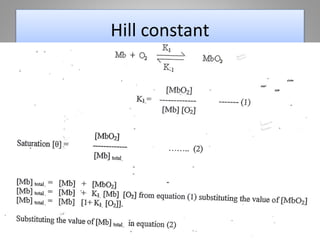
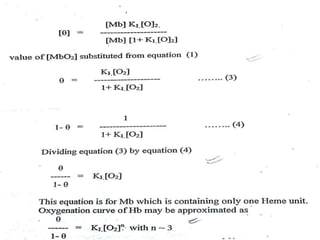
The document discusses the structure and function of hemoglobin, detailing its composition, synthesis, and historical research developments. It highlights key aspects such as oxygen binding, cooperative and allosteric effects, variants of hemoglobin, and associated genetic mutations. Additionally, it addresses hemoglobin deficiencies, diseases related to hemoglobin such as sickle cell disease, and mentions possible treatments.