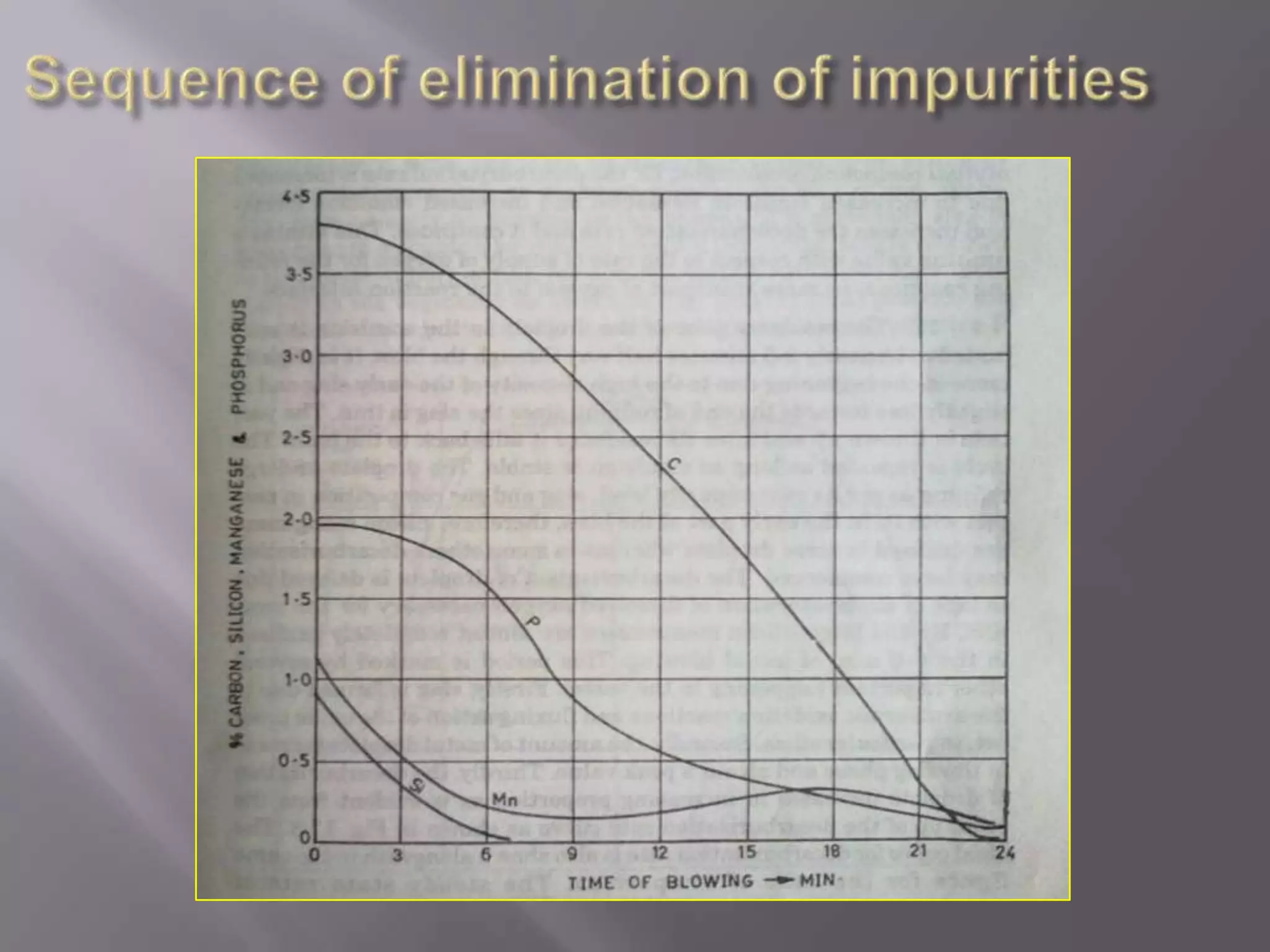
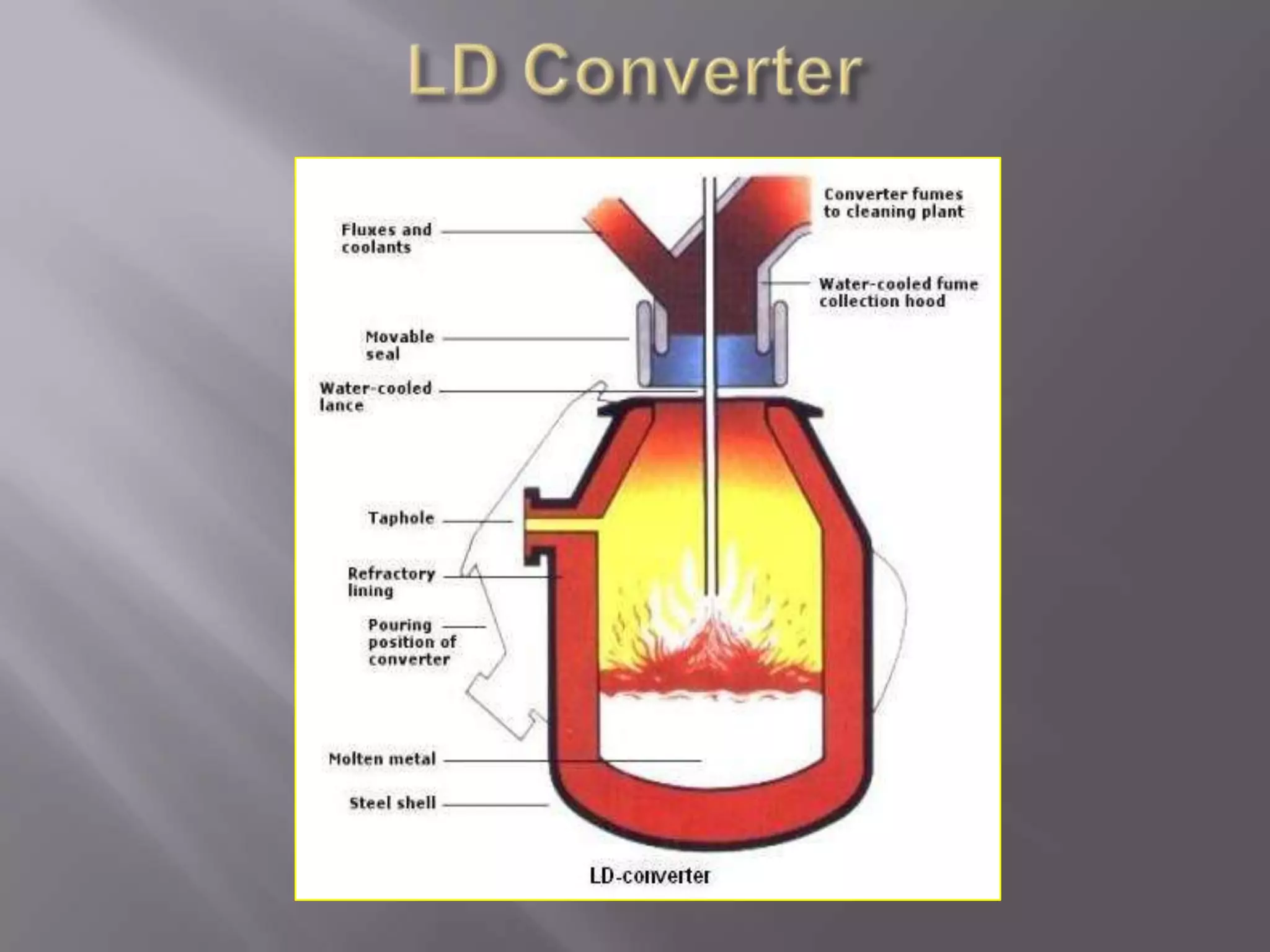
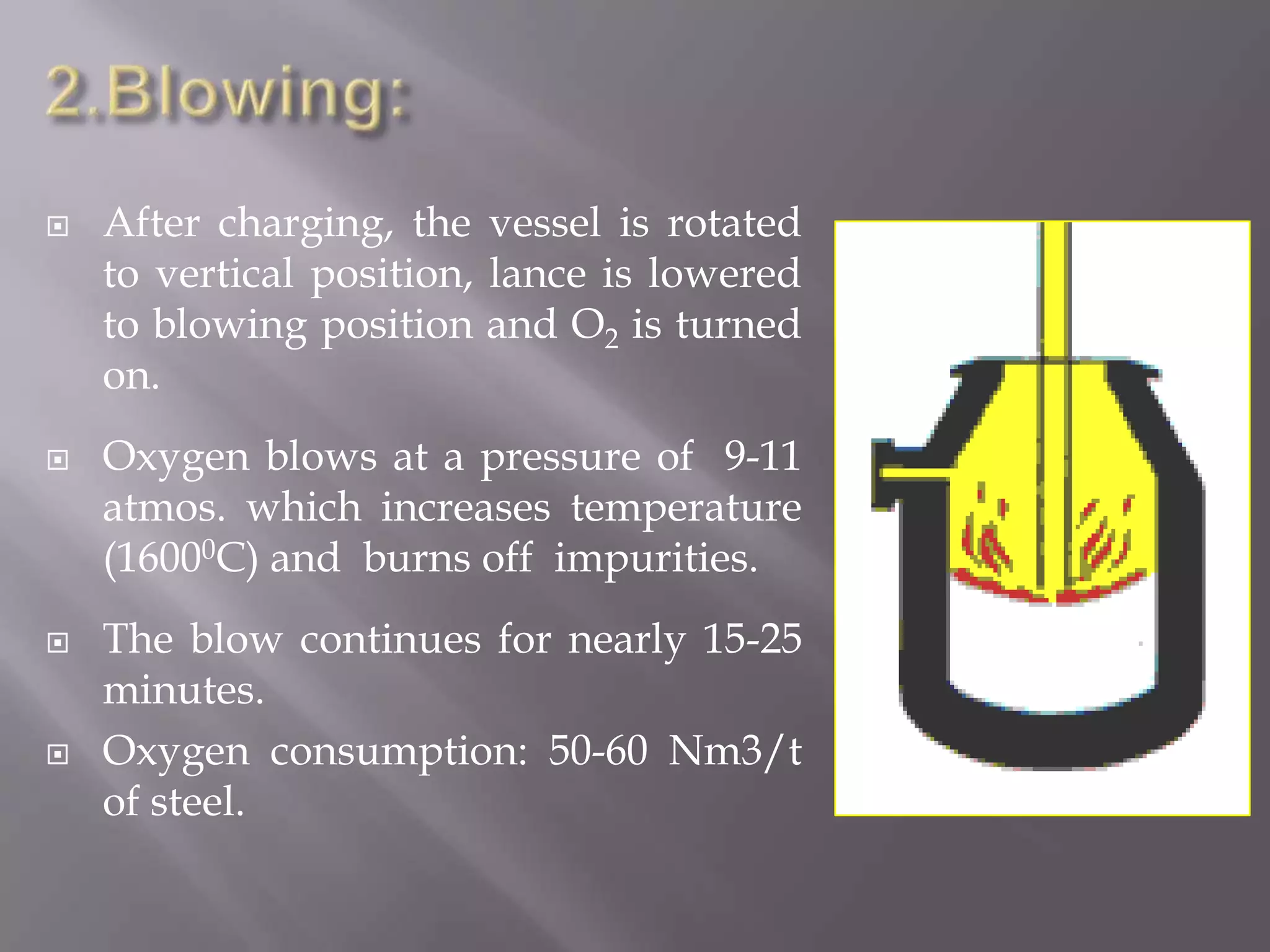
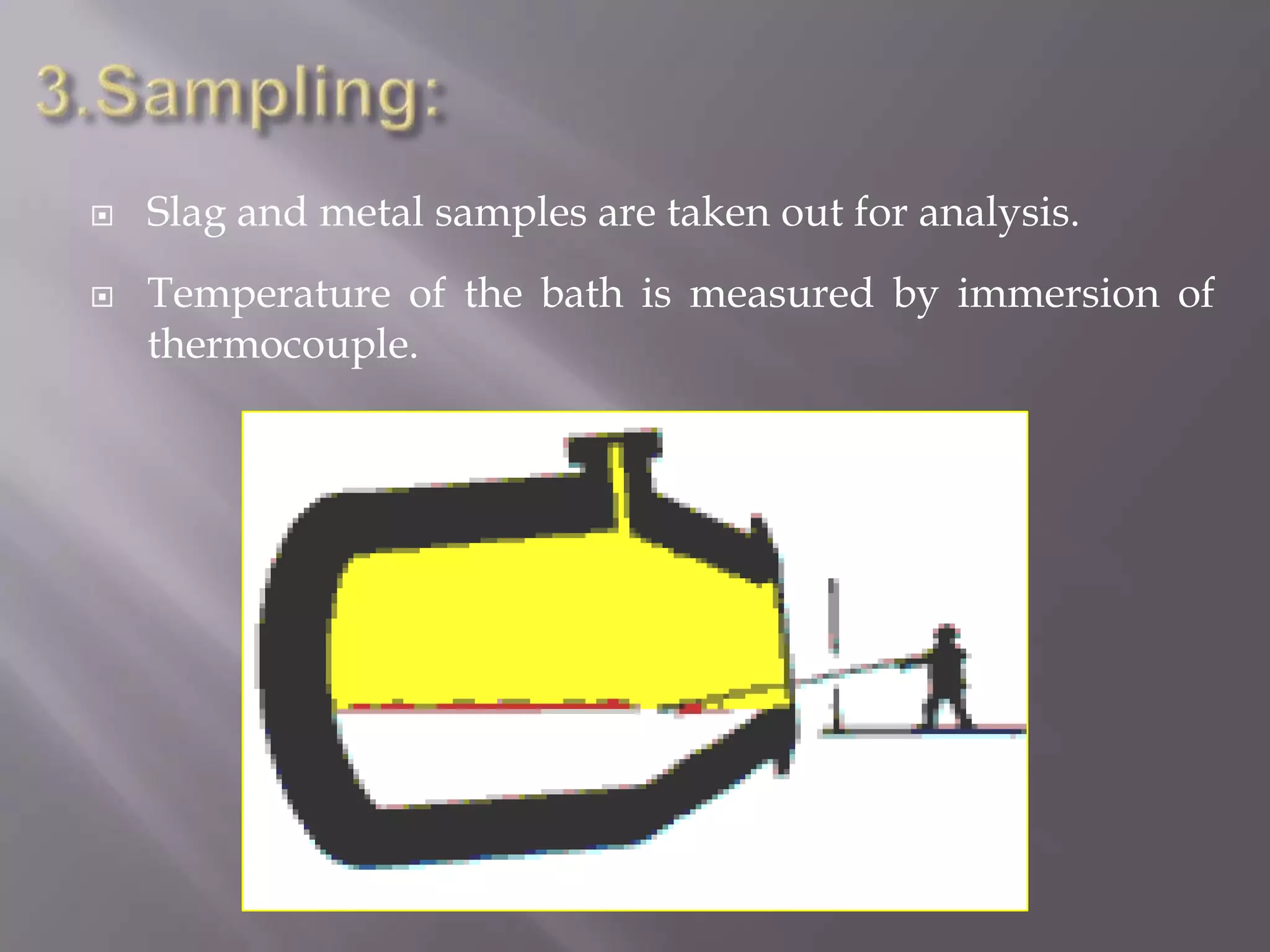
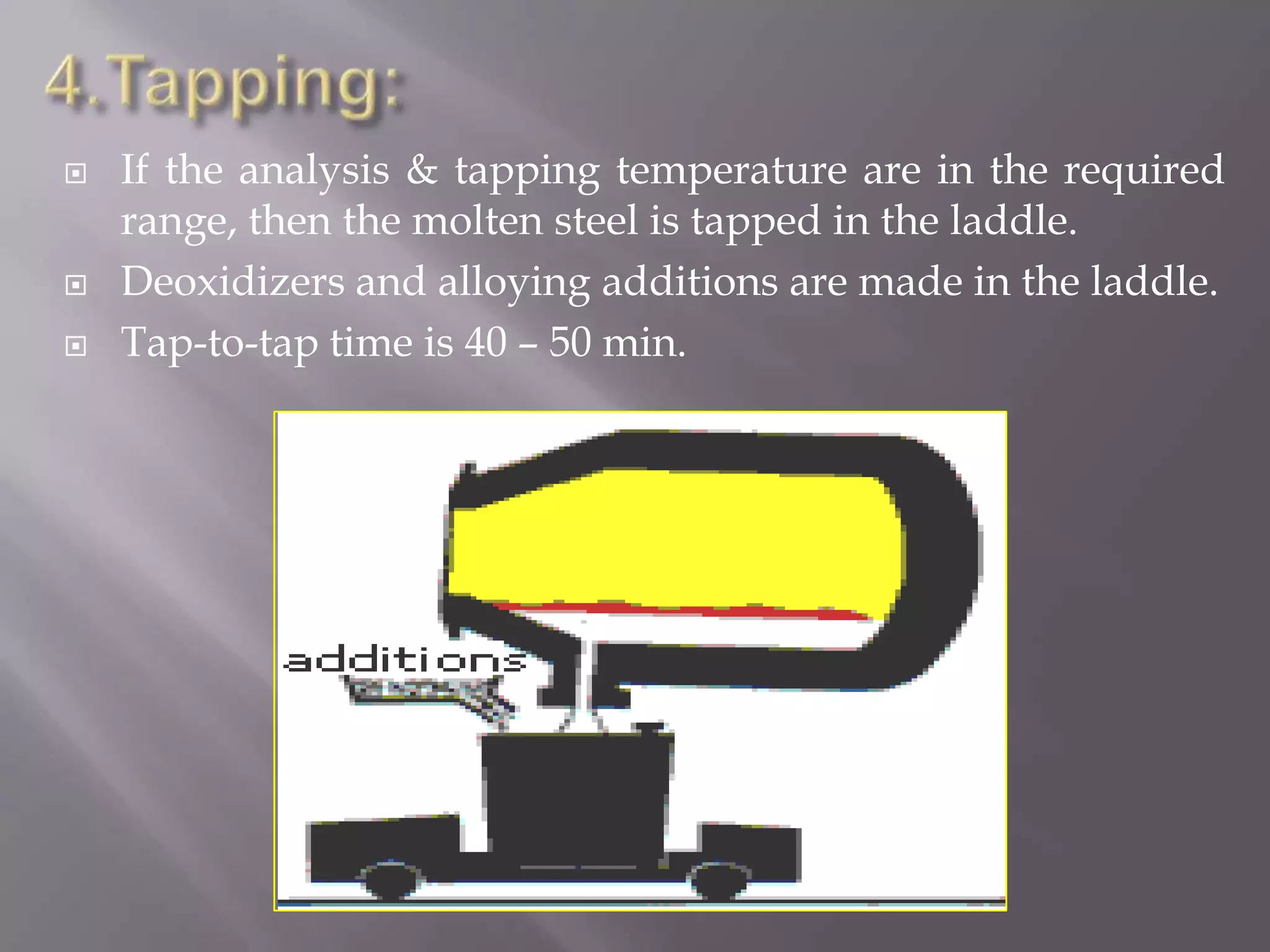
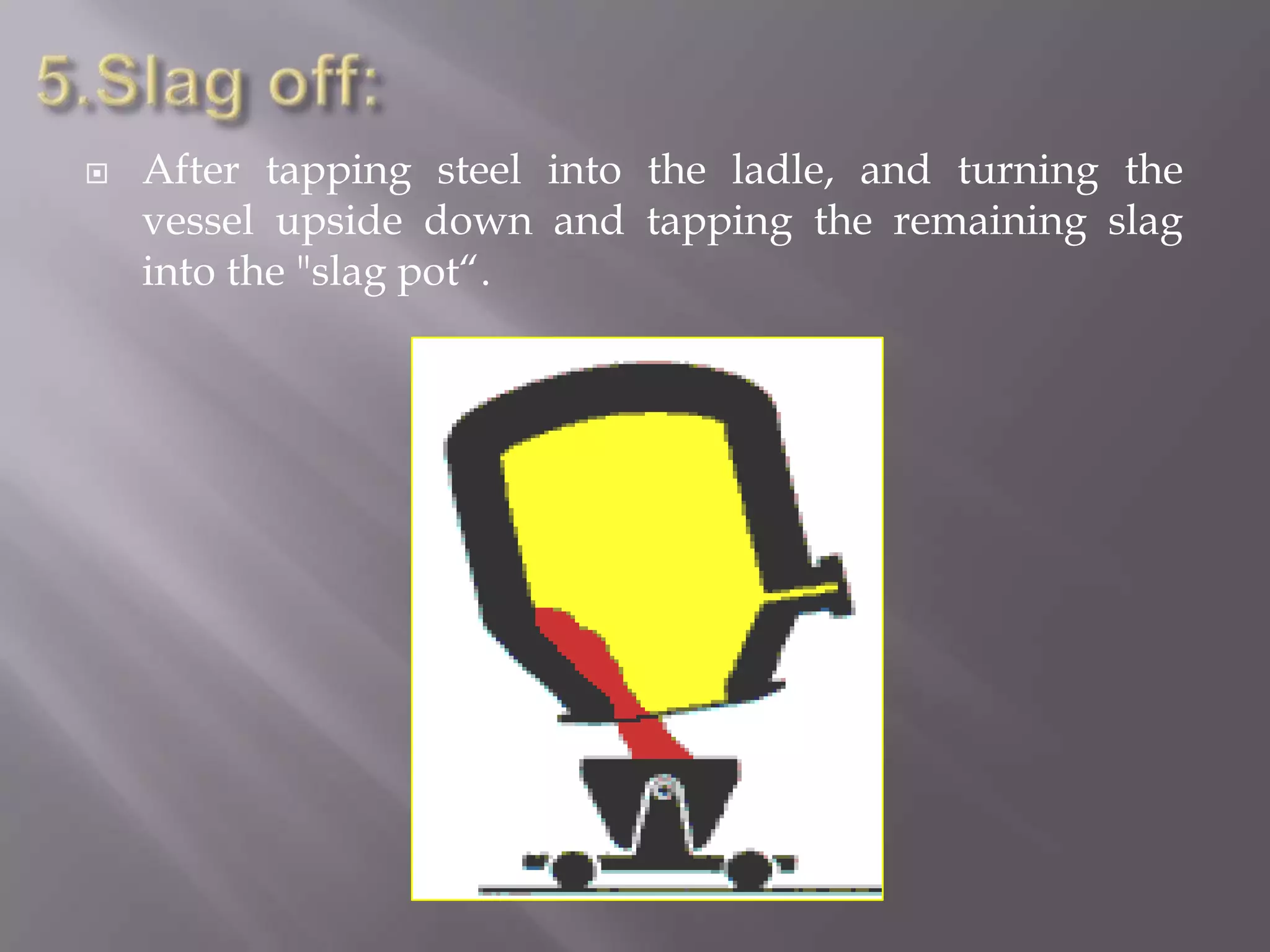
The LD process, also called the basic oxygen process, is a steelmaking method where scrap metal and iron ore are refined in an LD vessel. Key steps include charging materials, blowing oxygen through a lance at high pressure and temperature to burn off impurities, sampling the molten steel, and tapping purified steel into a ladle. The process is much faster than open hearth and produces steel with low sulfur and phosphorus using ordinary raw materials without external heat or fuel. However, it is limited in scrap usage and can result in steel wastage from splashing.








![1.
[Fe]
+
[O] = (FeO)
2.
[C]
+
[O] = {CO}
3.
[Si]
+
2 [O] = (SiO2)
4.
[Mn]
+
[O] = (MnO)
5.
2[P]
+
5[O] = (P2O5)
6.
[FeS/MnS]
+
(CaO) = (CaS) + (FeO/MnO)](https://image.slidesharecdn.com/ldprocess-140223031300-phpapp02/75/LD-process-14-2048.jpg)