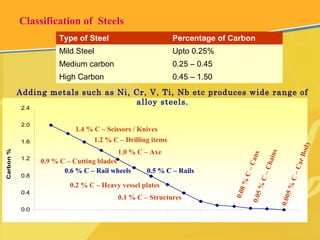
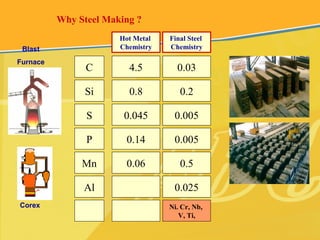
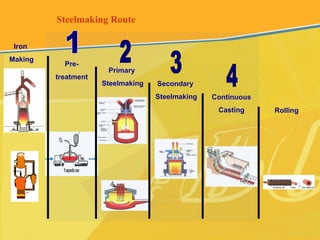
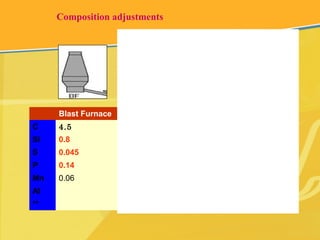
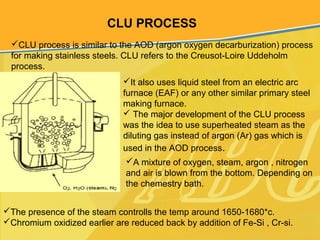
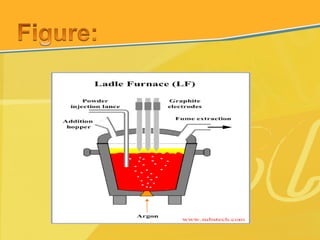
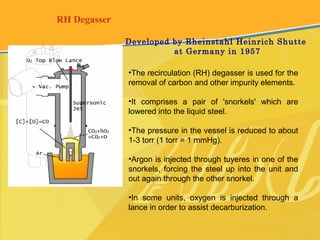
The document discusses different types and production processes of steel. It begins by introducing different types of steel based on carbon content, such as mild steel and alloy steels. It then describes the basic steelmaking route involving iron making, primary and secondary steelmaking, and continuous casting. The main secondary steelmaking processes discussed are AOD, VOD, CLU, ladle furnace treatment, and RH degassing. Each process's purpose and functioning are explained briefly.