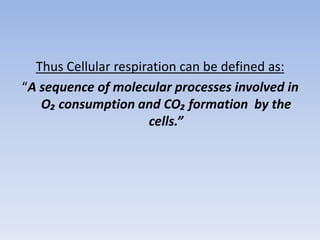
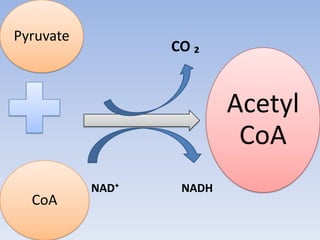
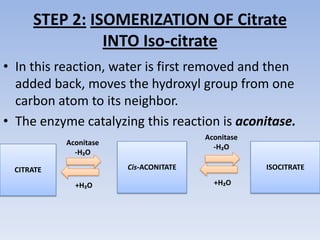
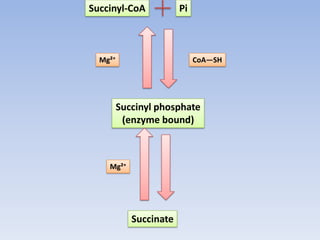
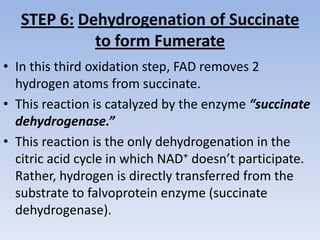
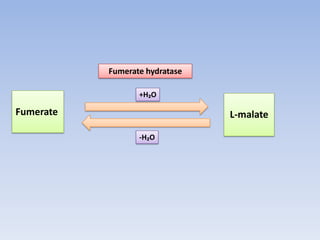
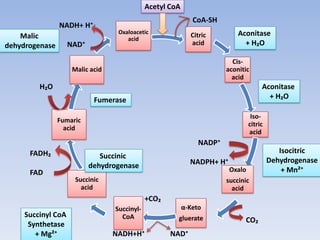
The document summarizes the three stages of cellular respiration - oxidative decarboxylation of pyruvate, the citric acid cycle (Krebs cycle), and the electron transport chain. It describes how pyruvate is converted to acetyl-CoA which feeds into the citric acid cycle. The citric acid cycle is a series of reactions that oxidizes acetyl-CoA completely, producing carbon dioxide and hydrogen carriers (NADH and FADH2) to be used in the final stage of oxidative phosphorylation.