This document provides an overview of conductometry, including:
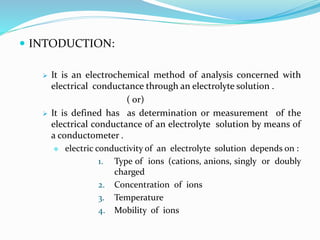
1. The principles of conductometry involve measuring the electrical conductance of an electrolyte solution using a conductometer. Conductance depends on ion type, concentration, temperature, and mobility.
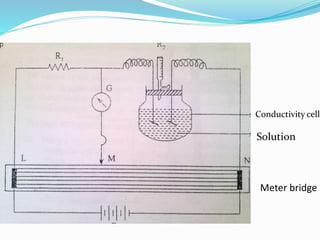
2. Instrumentation includes a current source, conductivity cells with platinum electrodes, and a conductance bridge to measure resistance and calculate conductivity.
3. Conductometric titrations can be used for acid-base, redox, precipitation, and complexometric titrations. They do not require indicators and can be used for colored or turbid solutions.