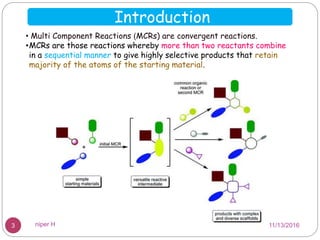
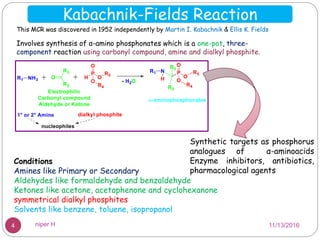
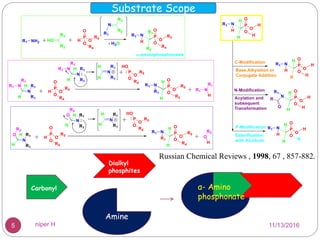
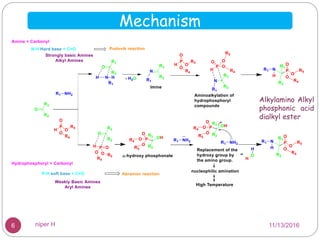
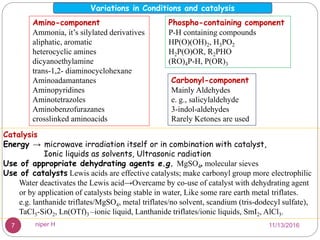
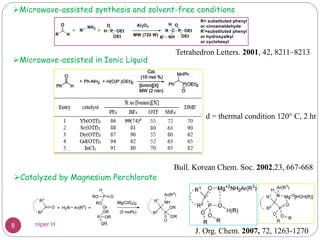
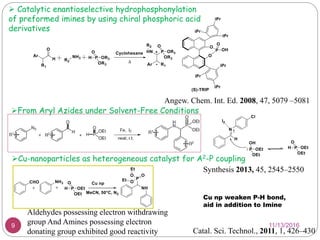
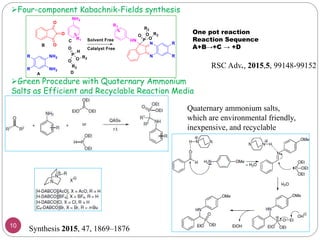
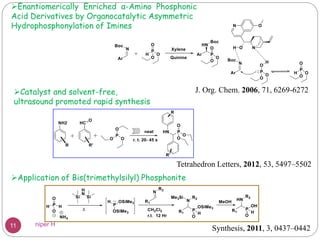
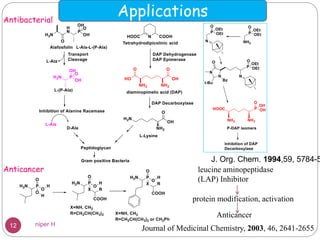
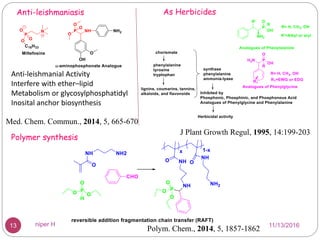
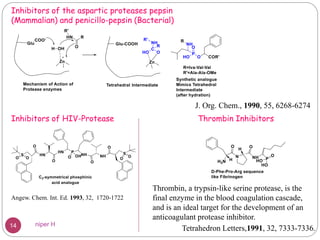
The Kabachnik-Fields reaction is a multi-component reaction that involves the synthesis of α-amino phosphonates from a carbonyl compound, amine, and dialkyl phosphite. It was discovered independently in 1952 by Martin I. Kabachnik and Ellis K. Fields. The reaction produces α-amino phosphonate products in a one-pot reaction under mild conditions. Variations to the traditional Kabachnik-Fields reaction include using different substrates, catalysts, solvents and conditions such as microwave irradiation or solvent-free systems. The α-amino phosphonate products have applications as enzyme inhibitors, antibiotics, and in polymer synthesis.