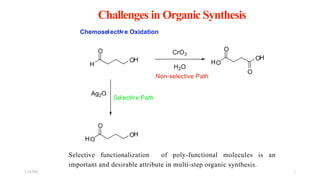
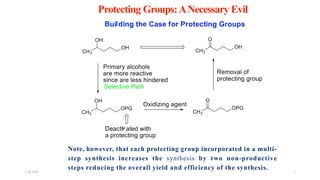
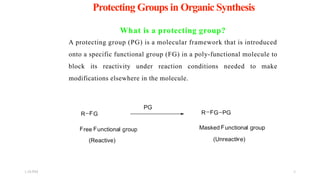
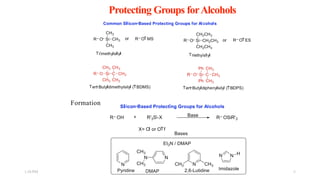
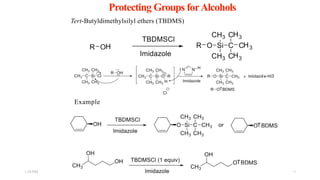
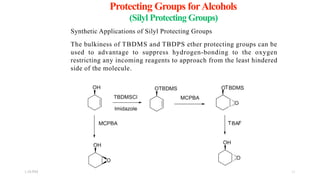
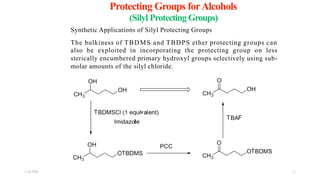
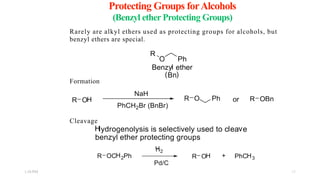
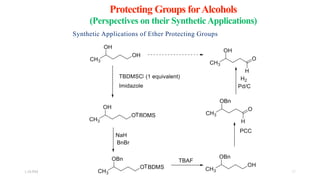
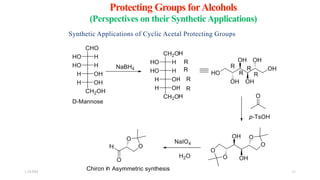
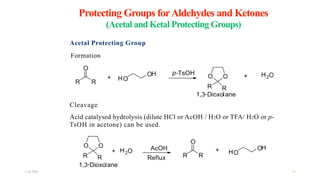
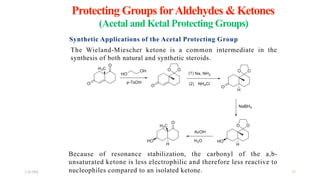
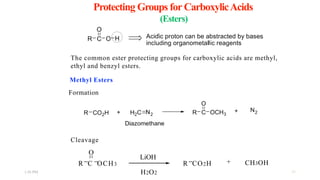
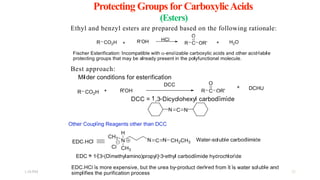
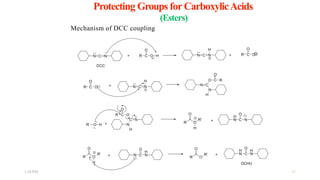
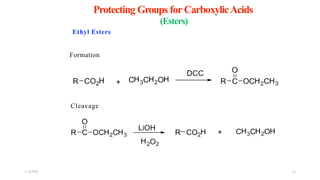
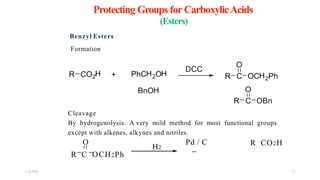
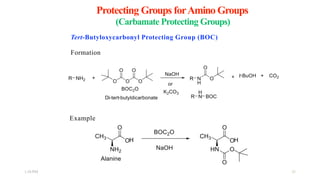
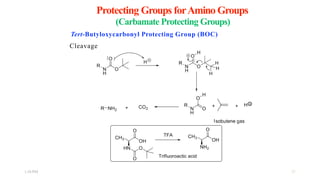
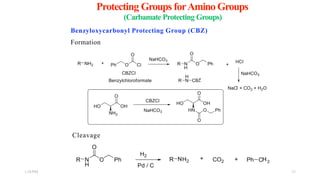
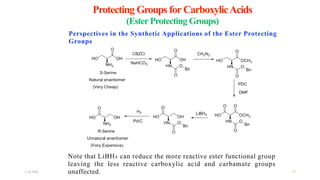
This document discusses protecting groups used in organic synthesis. Protecting groups are introduced to block the reactivity of functional groups during multi-step synthesis. Common protecting groups discussed include silyl, acetal, and ester protecting groups for alcohols, ketals and acetals for aldehydes and ketones, esters for carboxylic acids, and carbamates for amino groups. The qualities of good protecting groups and their selective introduction and removal are explained.