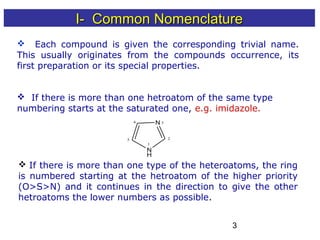
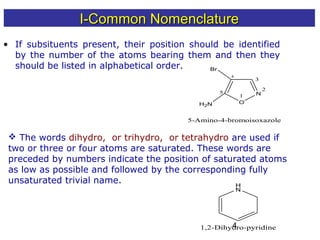
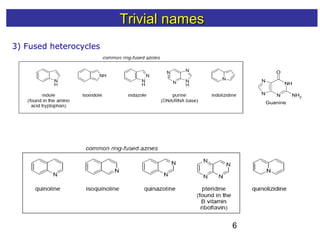
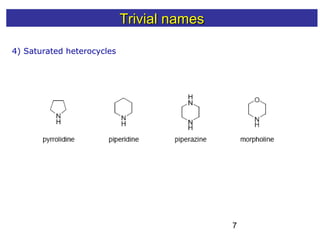
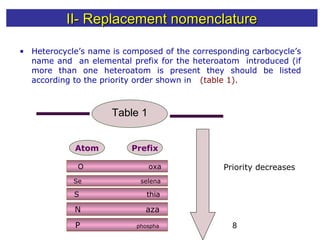
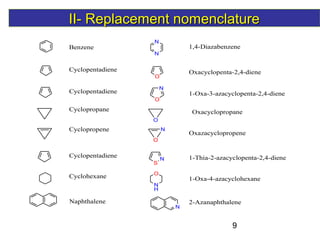
1) The document discusses various systems for naming heterocyclic compounds, including common nomenclature, replacement nomenclature, and Hantzsch-Widman (IUPAC) nomenclature.
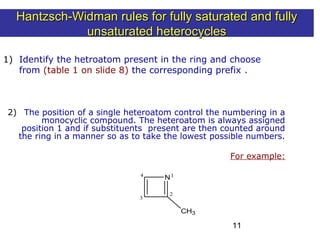
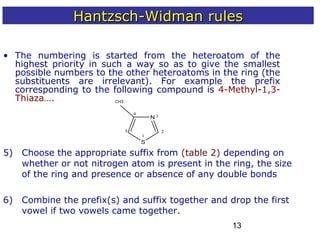
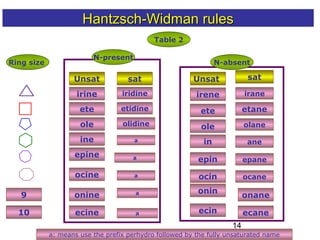
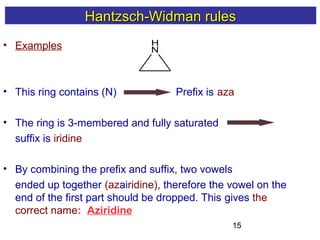
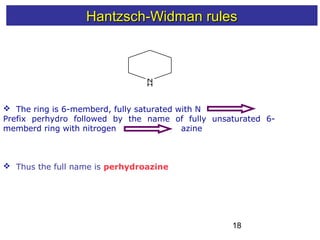
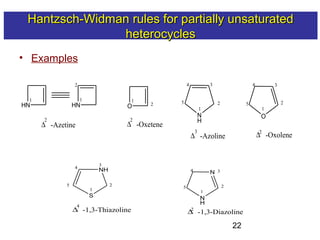
2) The Hantzsch-Widman system involves identifying the heteroatom prefixes, assigning ring positions starting with the highest priority heteroatom, choosing a suffix based on ring size and saturation, and combining them while dropping repeated vowels.
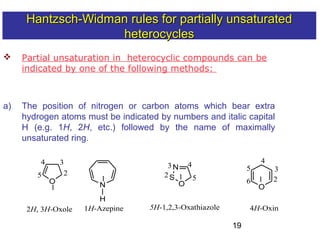
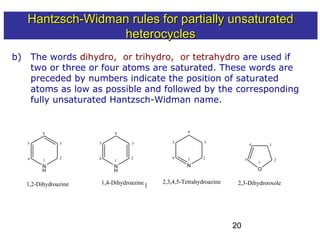
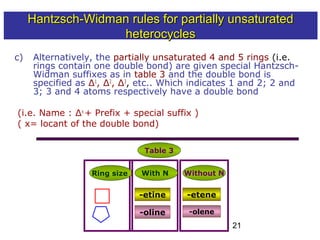
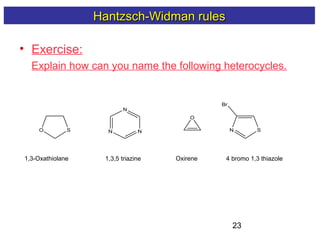
3) Rules are provided for naming monocyclic, polycyclic, saturated, partially unsaturated, and compounds with multiple heteroatoms. Locants are used to indicate substituent and double bond positions.