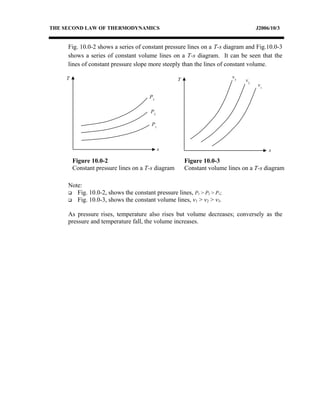
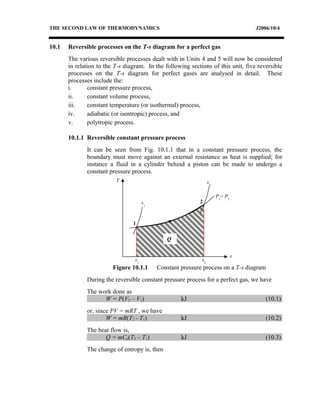
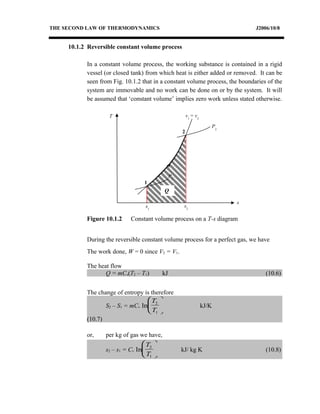
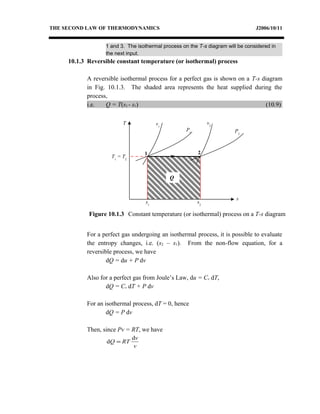
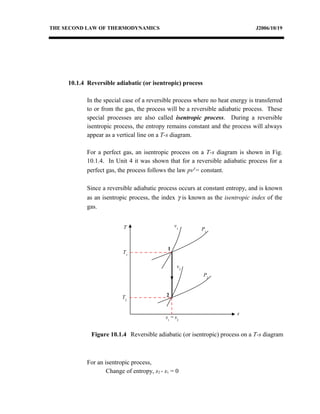
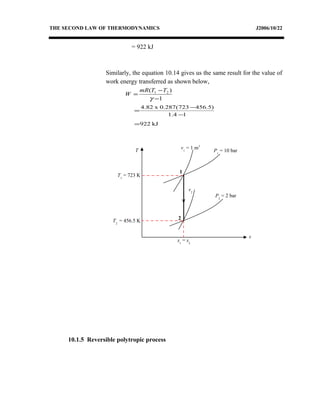
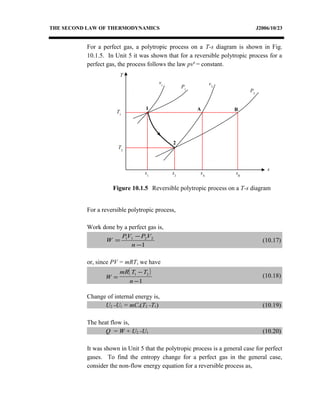
The document discusses the second law of thermodynamics and various reversible processes on a temperature-entropy (T-s) diagram for a perfect gas. It defines:
1) Constant pressure, volume, temperature, adiabatic, and polytropic processes on a T-s diagram.
2) Equations to calculate work, heat, and entropy change for constant pressure, volume, and temperature processes.
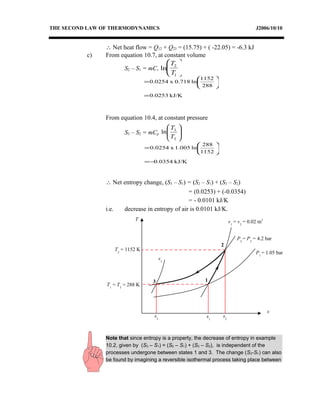
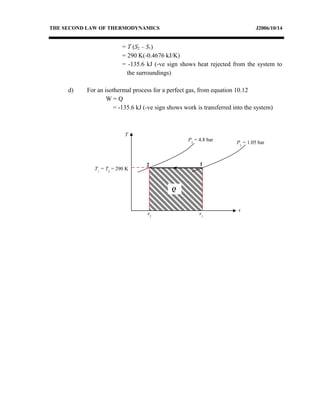
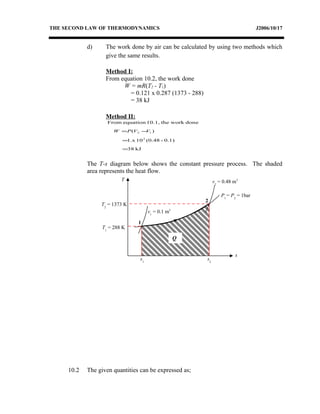
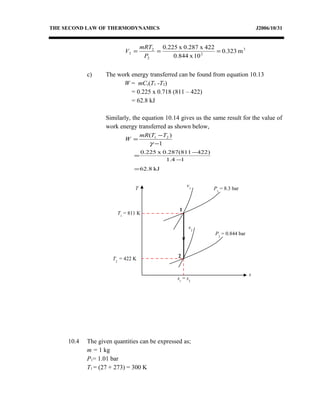
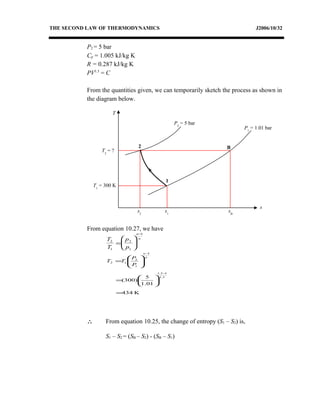
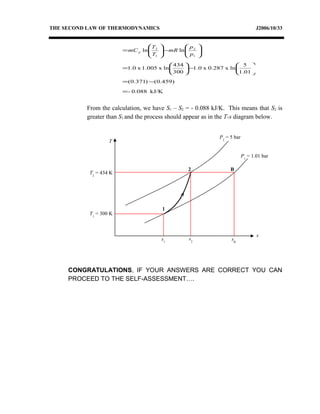
3) Provides an example problem calculating properties of air undergoing two processes - constant volume heating and constant pressure cooling.