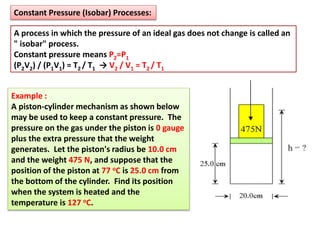
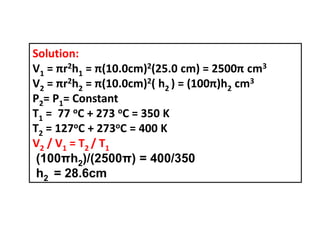
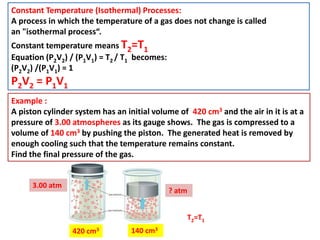
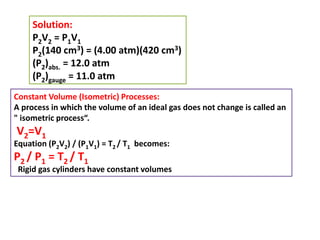
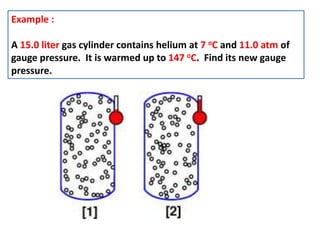
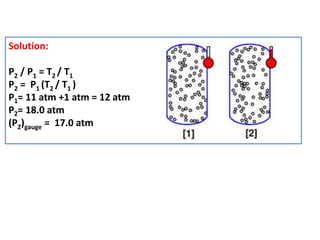
This document discusses the expansion of gases and the relationships between pressure, volume, temperature, and number of moles in gases. It introduces the ideal gas law (PV=nRT) and describes some key gas processes including isobaric (constant pressure), isothermal (constant temperature), and isometric (constant volume) processes. Examples are provided to demonstrate how to use the ideal gas law and gas equations of state to calculate pressure, volume, temperature, or number of moles given values of the other variables.

![Example :
a) Calculate the average K.E. of air molecules at 27.0 oC.
b) Calculate the average speed of its constituents (oxygen molecules and
nitrogen molecules).
1 mole of O2 = 32.0 grams= 6.02x1023 molecules of O2
1 mole of N2 = 28.0 grams= 6.02x1023 molecules of N2
Solution:
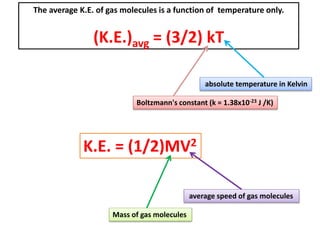
K.E. = (3/2) k T
K.E. = (3/2) (1.38x10-23 J/K)(27+273)K = 6.21x10-21 J/molecule
(Every gas molecule at this temperature, has 6.21x10-21 J whether it is a
single O2 molecule or a single N2 molecule).
For every O2 molecule: K.E. = (1/2)MV2
6.21x10-21 J = (1/2) [ 32.0x10-3 kg / 6.02x1023]V2 → V = 483m/s
For every N2 molecule: K.E. = (1/2)MV2
6.21x10-21 J = (1/2) [ 28.0x10-3 kg / 6.02x1023 ]V2 → V = 517m/s
Each N2 molecule is lighter; therefore, its average speed is
higher. Each O2 molecule is heavier; therefore, its average speed is lower at
the same temperature.](https://image.slidesharecdn.com/chapter14-141115172507-conversion-gate02/85/Chapter-14-4-320.jpg)
![Expansion of Gases
Perfect Gas Law:
PV = nRT
gas absolute pressure
V is its volume (the volume of its container)
Universal gas constant = 8.314 [J/(mole K)]
gas absolute temperature in Kelvin
The two conditions for a gas to be ideal or obey this equation are:
1) The gas pressure should not exceed about 8 atmospheres.
2) The gas must be superheated (gas temperature sufficiently
above its boiling point) at the operating pressure and volume.
number of moles of gas
Equation of State](https://image.slidesharecdn.com/chapter14-141115172507-conversion-gate02/85/Chapter-14-5-320.jpg)
![The Unit of " PV ":
Note that the product " PV " has dimensionally the unit of "energy “.
In SI, the unit of "P" is [ N / m2 ] and the unit of volume " V " is [ m3 ].
On this basis, the unit of the product " PV " becomes [ Nm ] or [ Joule ].
The " Joule " that appears in R = 8.314 J /(mole K) is for this reason.
Example :
A 0.400 m3 tank contains nitrogen at 27 oC. The pressure gauge on it reads
3.75 atmosphere. Find
(a) the number of moles of gas in the tank
(b) its mass in kg.](https://image.slidesharecdn.com/chapter14-141115172507-conversion-gate02/85/Chapter-14-6-320.jpg)
![Solution:
Pabs. = Pgauge + 1 atm = 4.75 atm
Tabs. = 27oC + 273 = 300K
PV = nRT → n = (PV) / [RT]
n = (4.75x101000Pa)(0.400m3) / [(8.314 J / (mole K))300K]
(a) n = 76.9 moles
(b) M = (76.9 moles)(28.0 grams /mole) = 2150 grams = 2.15 kg](https://image.slidesharecdn.com/chapter14-141115172507-conversion-gate02/85/Chapter-14-7-320.jpg)
![Example :
A 0.770 m3 hydrogen tank contains 0.446 kg of hydrogen at 127 oC.
The pressure gage on it is not working. What pressure should the gauge show?
Each mole of H2 is 2.00 grams.
Solution:
n = (0.446x103 grams) / (2.00 grams / mole) = 223 moles
PV = nRT → P = (nRT) / V
P = (223 moles)[ 8.314 J/(mole K)] (127 + 273)K / (0.770 m3)
Pabs = 963,000 Pa
Pgauge = Pabs - 1atm = 963,000 Pa - 101,000Pa = 862,000Pa (≈ 8.6 atm)](https://image.slidesharecdn.com/chapter14-141115172507-conversion-gate02/85/Chapter-14-8-320.jpg)
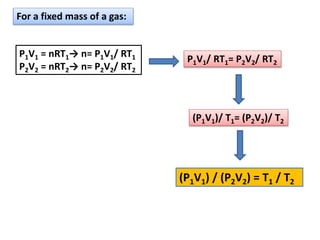
K /(3.80x101,000)Pa = 0.442m3
(b) (P2V2)/(P1V1) =T2 /T1
(7.6atm)(V2) /[(3.8atm)(0.442m3)] = 300K/400K
V2 = 0.166m3](https://image.slidesharecdn.com/chapter14-141115172507-conversion-gate02/85/Chapter-14-10-320.jpg)