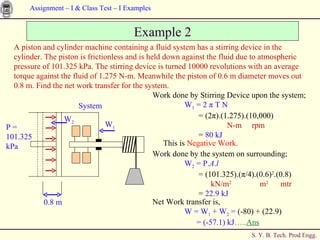
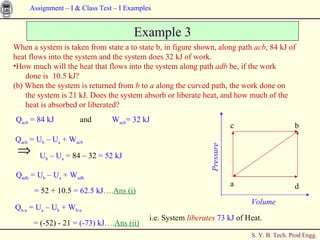
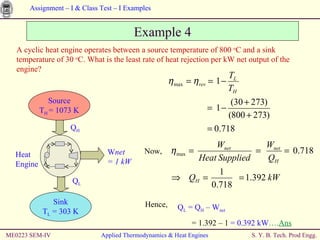
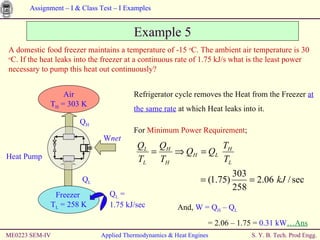
The document contains 7 examples of thermodynamics calculations involving concepts like steam tables, work, heat, ideal gases, refrigeration cycles, and processes involving gases. The examples calculate things like inlet and outlet steam pressures, net work done by systems, heat transferred in cycles, minimum heat rejection rate, refrigeration power requirement, work done by compressing an ideal gas, and net work in a sequence of gas processes.
![ME0223 SEM-IV Applied Thermodynamics & Heat Engines Example 1 A turbine is supplied with steam at a gauge pressure of 1.4 MPa. After expansion in the turbine, the steam flows into a condenser which is maintained at a vacuum of 710 mm of Hg. The barometric pressure is 772 mm Hg. Express the inlet and exhaust steam pressures in Pascal (absolute).Take the density of mercury as 13.6 X 10 3 kg/m 3 . The Atmospheric Pressure, P 0 = ρ .g.z 0 = (13.6 X 10 3 ).(9.81).(0.772) kg/m 3 m/sec 2 mtr = 1.03 X 10 5 Pa Inlet Steam Pressure, P i = [(1.4 X 10 6 ) + (1.03 X 10 5 )] Pa = 15.05 X 10 5 Pa = 1.503 MPa ….. Ans Outlet Steam Pressure, (i.e. Condenser Pressure) P 0 = (0.772 – 0.710).(9.81).(13.6 X 103) mtr m/sec 2 kg/m 3 = 8.27 kPa ….. Ans](https://image.slidesharecdn.com/seprodthermoexamplesassignmentclasstest-110220042736-phpapp01/85/Thermodynamics-Examples-and-Class-test-2-320.jpg)