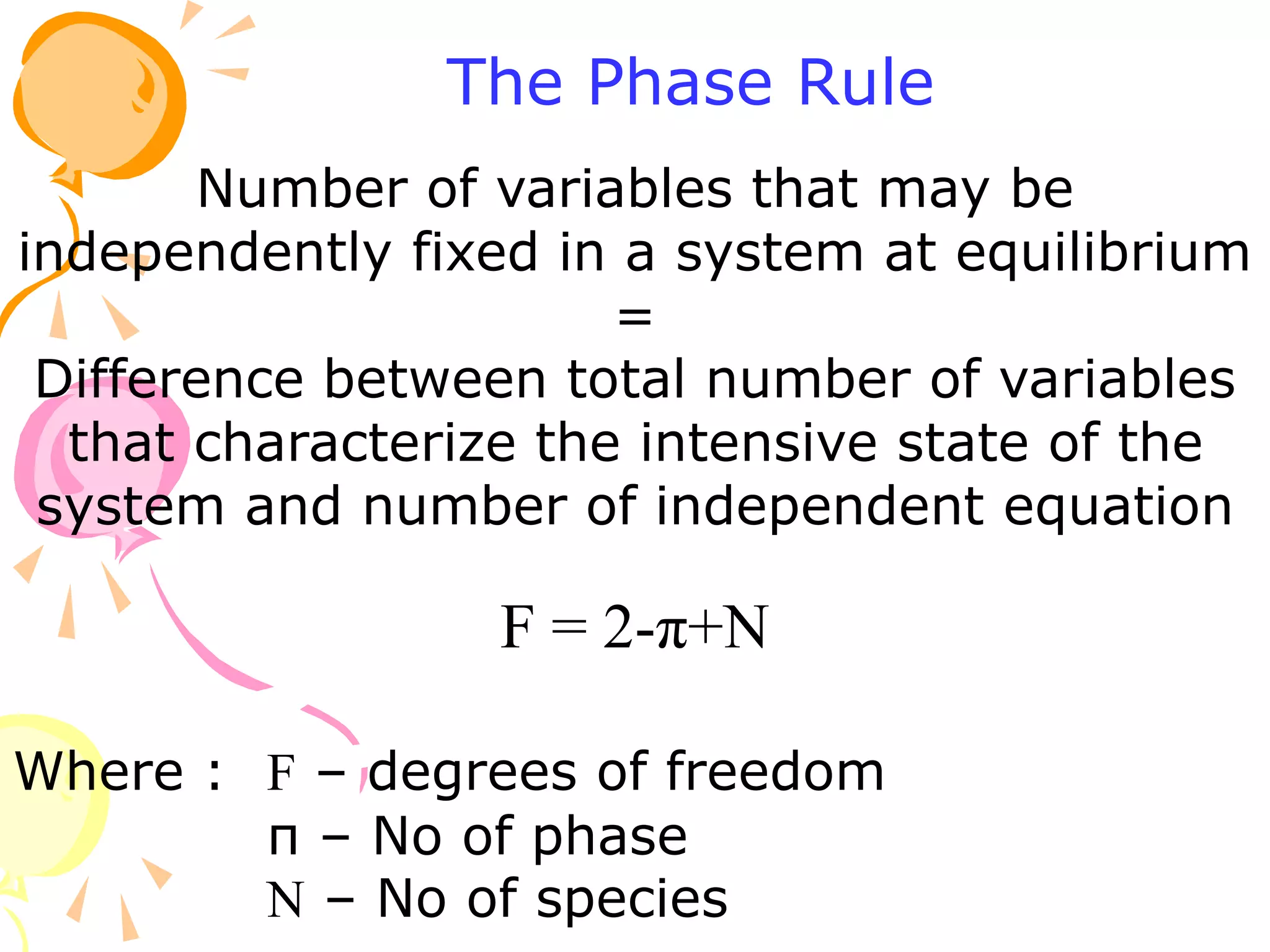
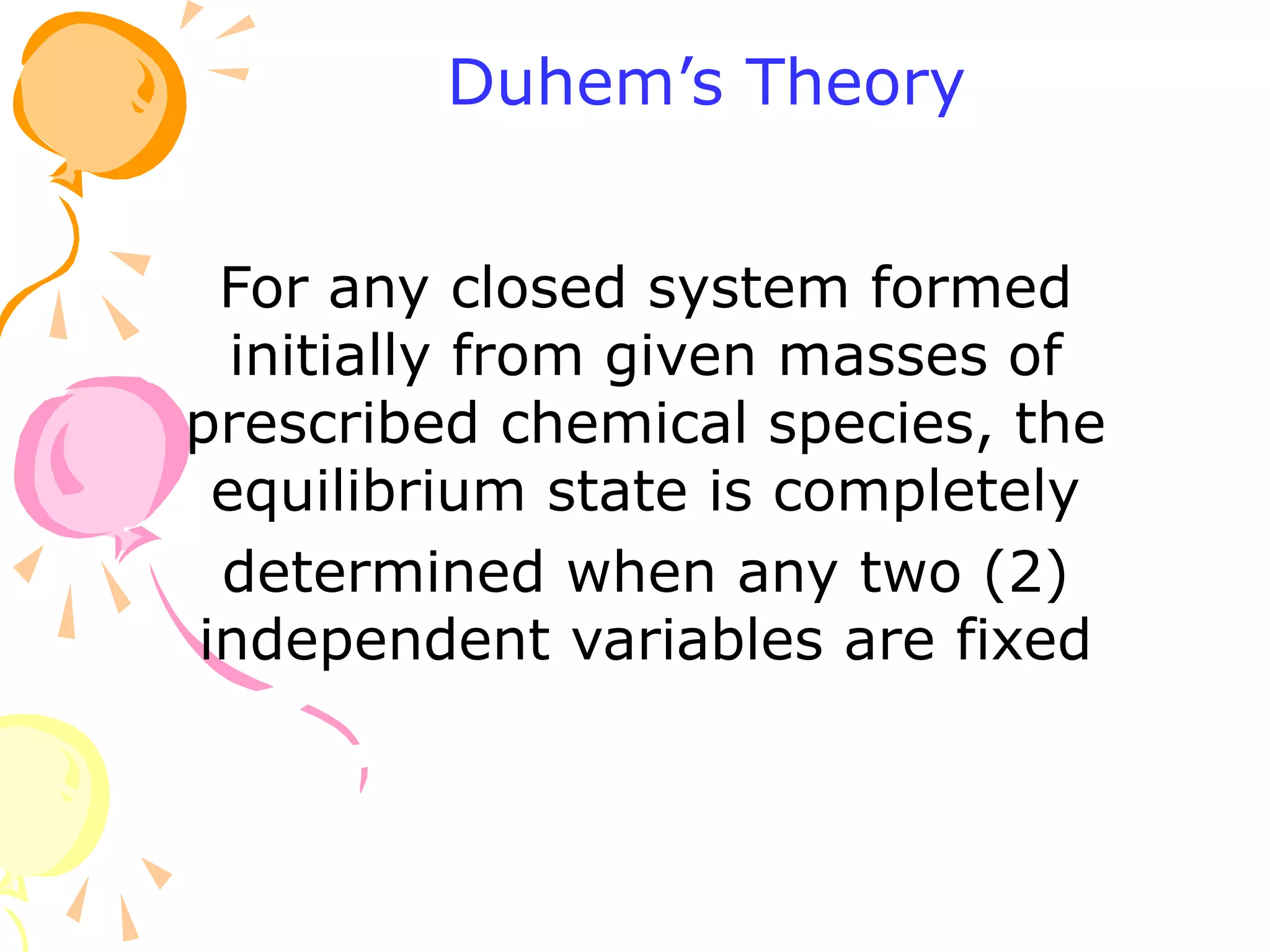
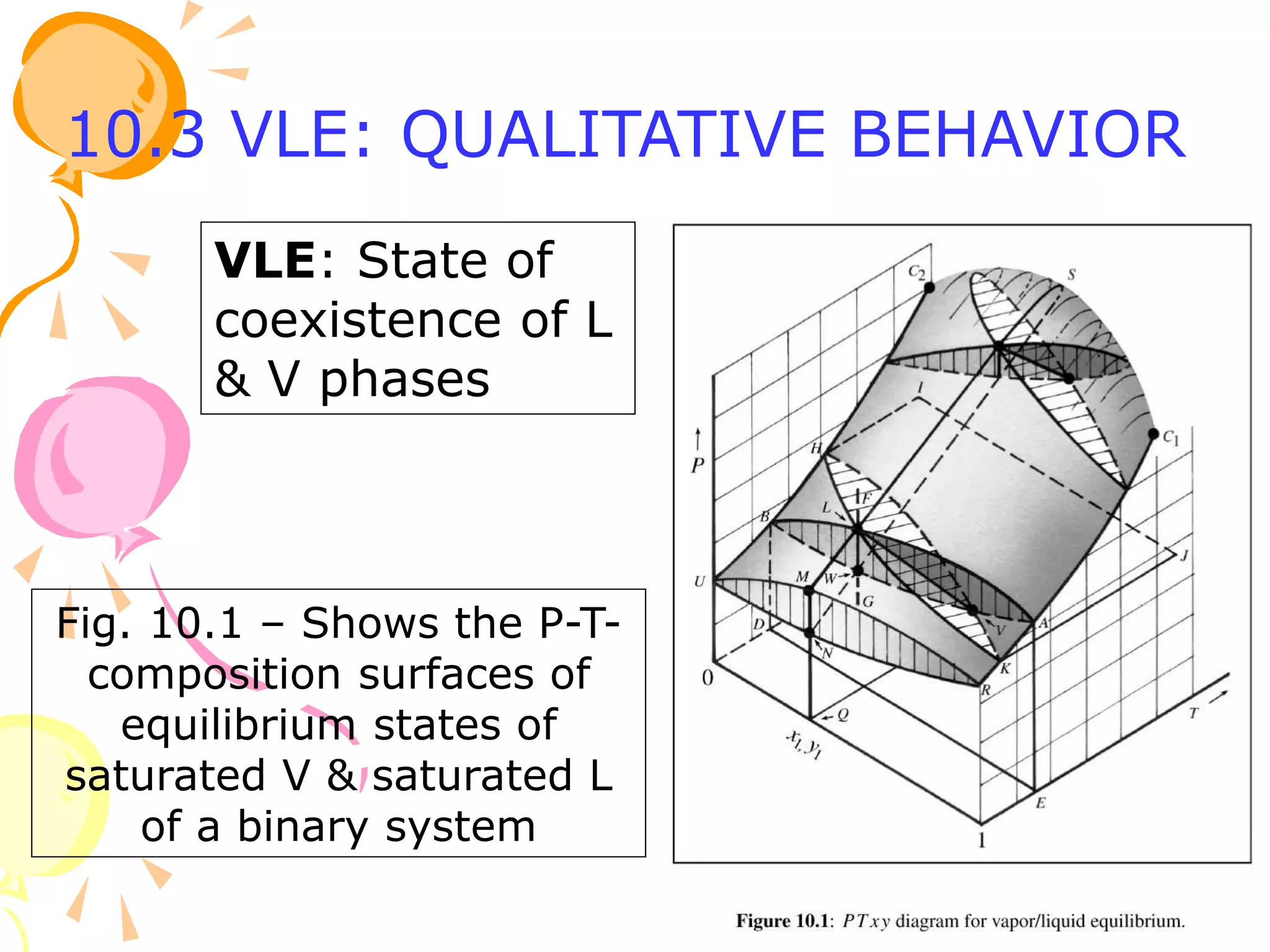
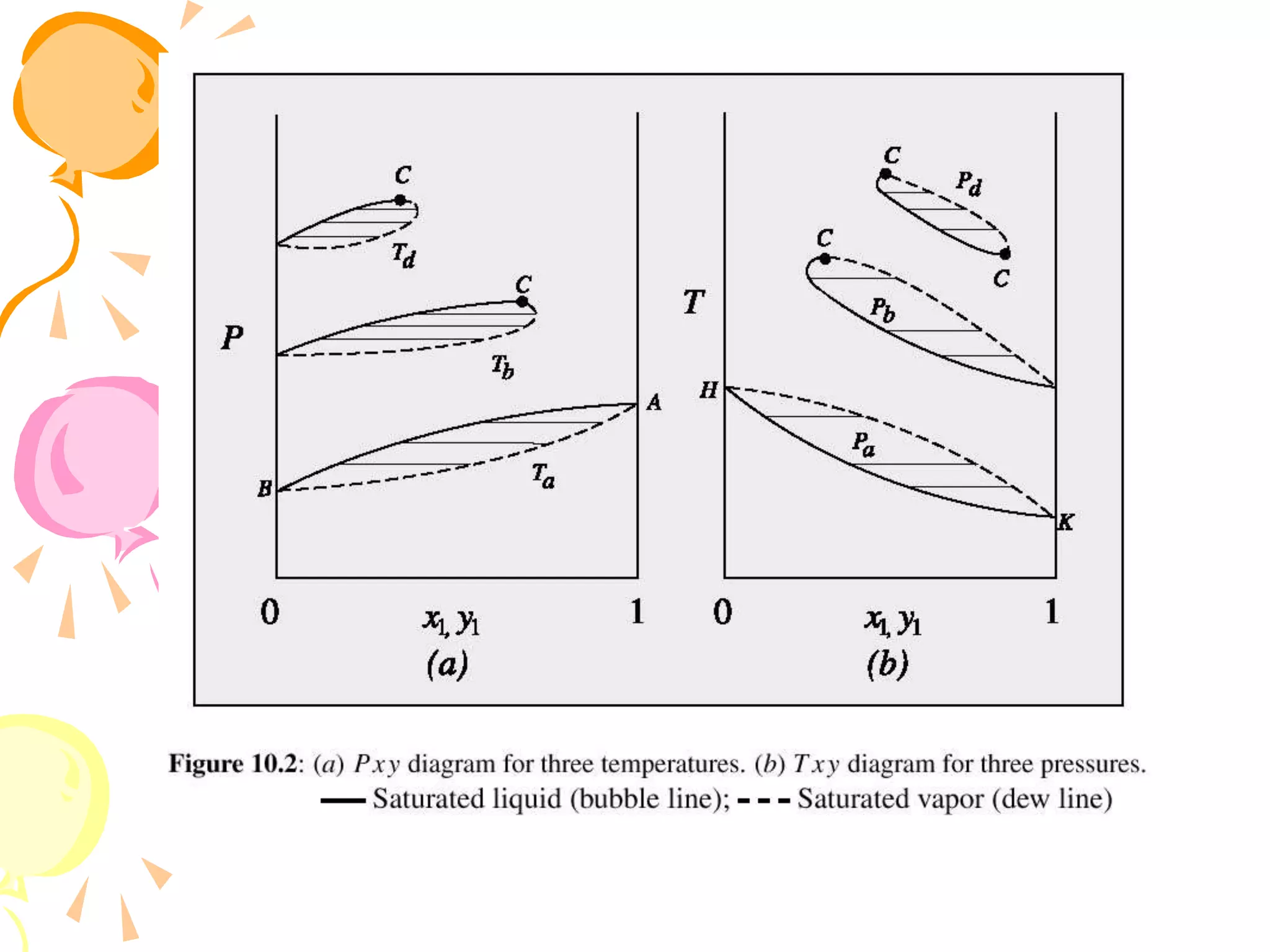
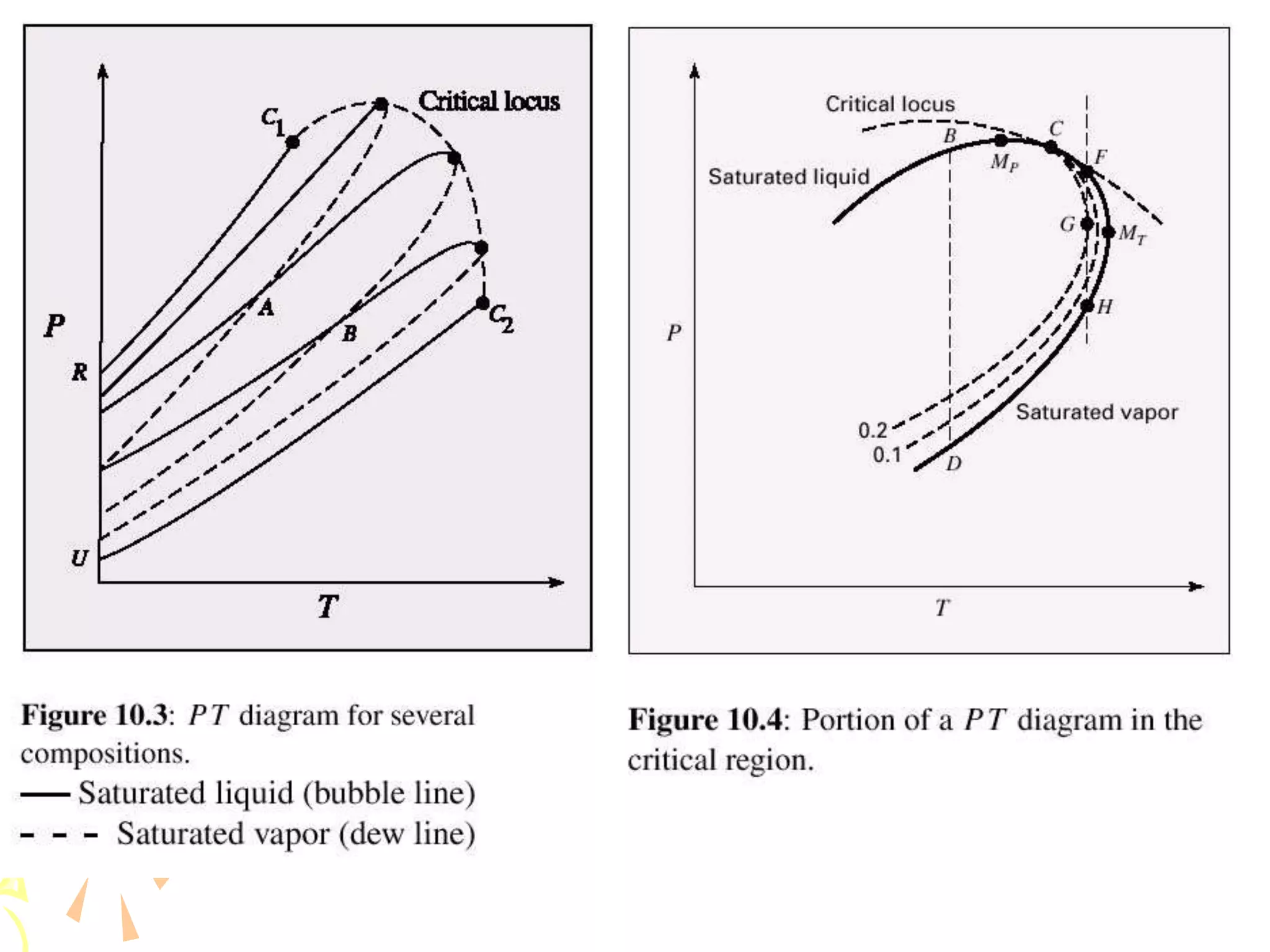
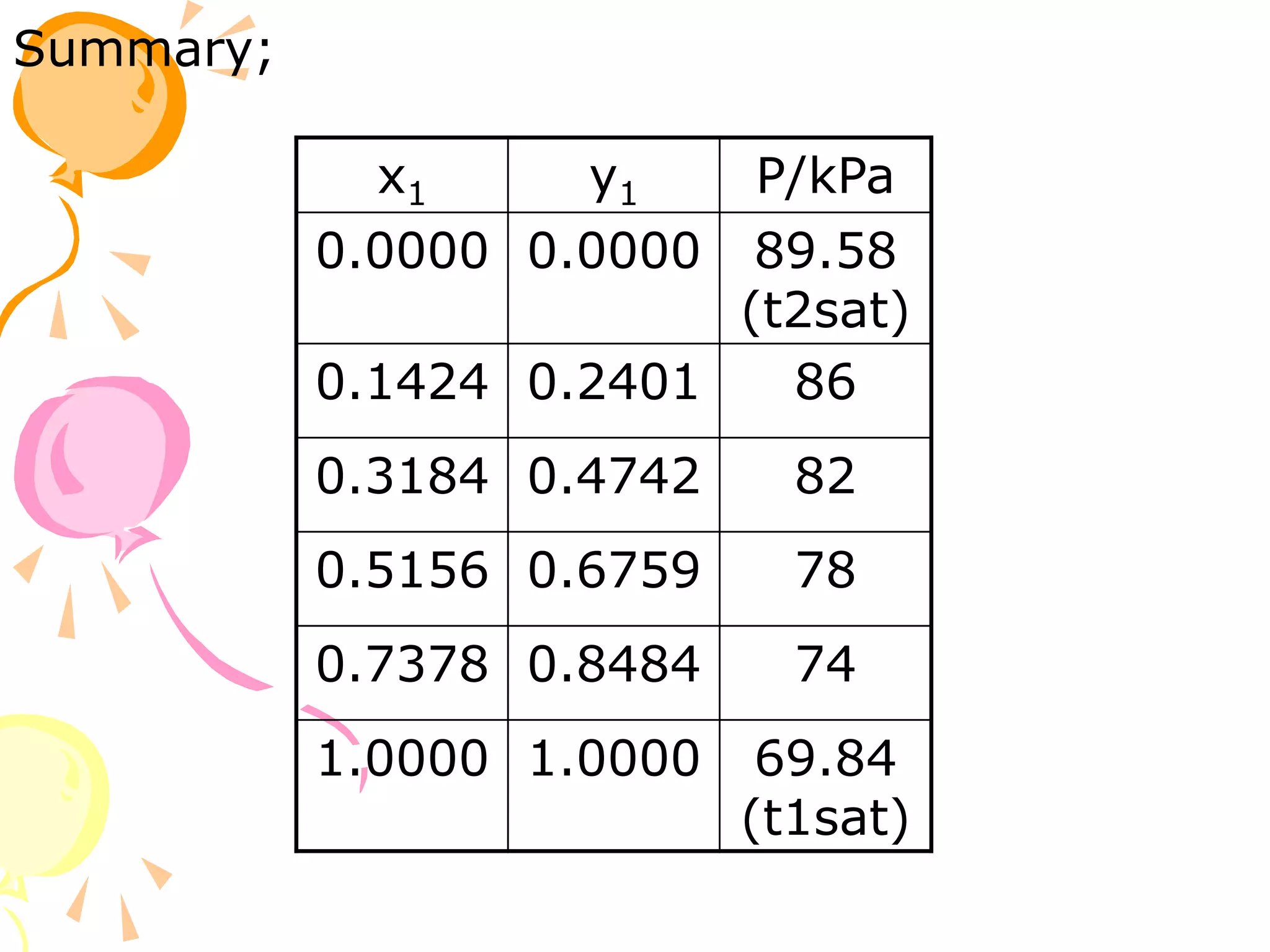
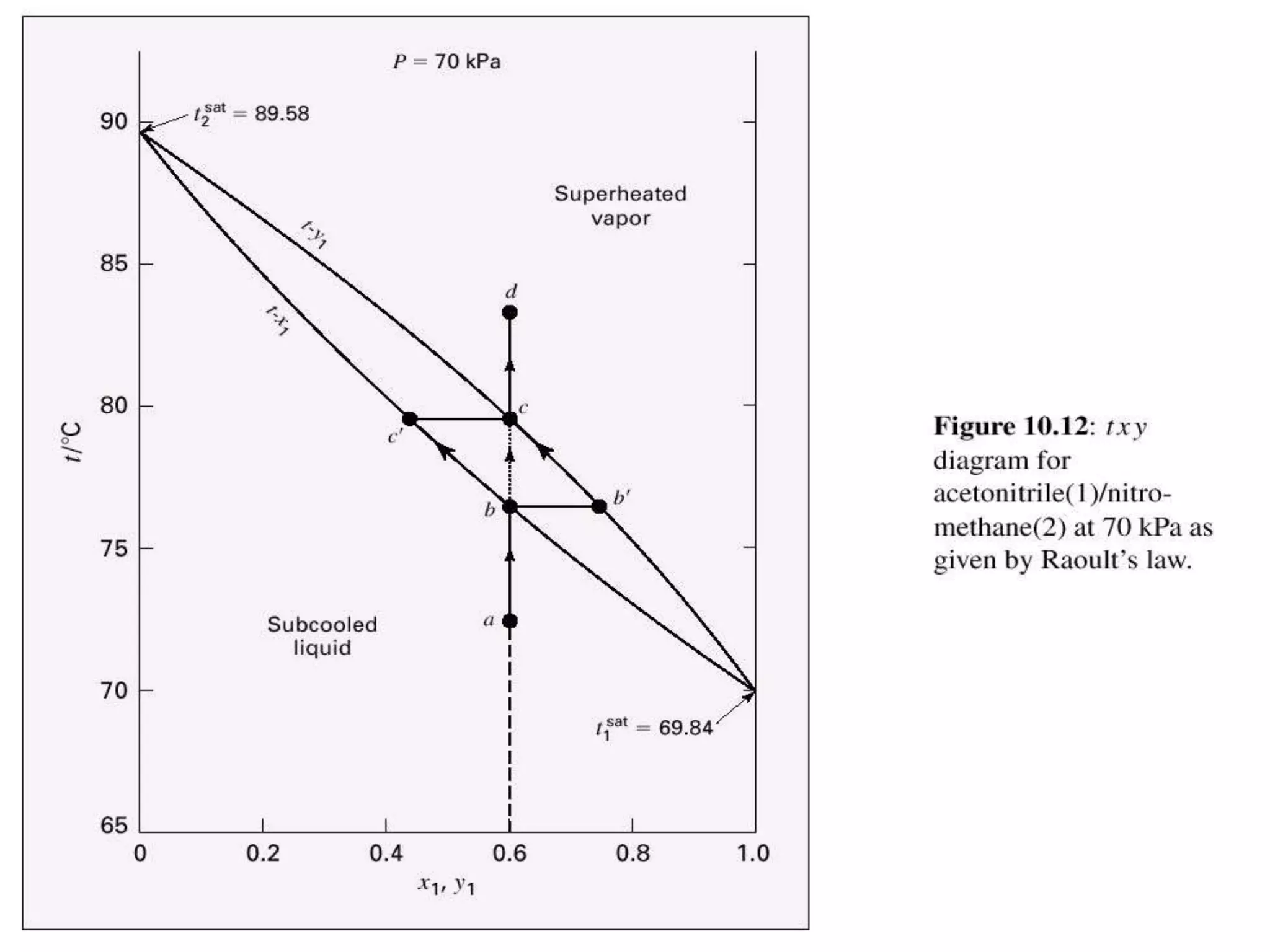
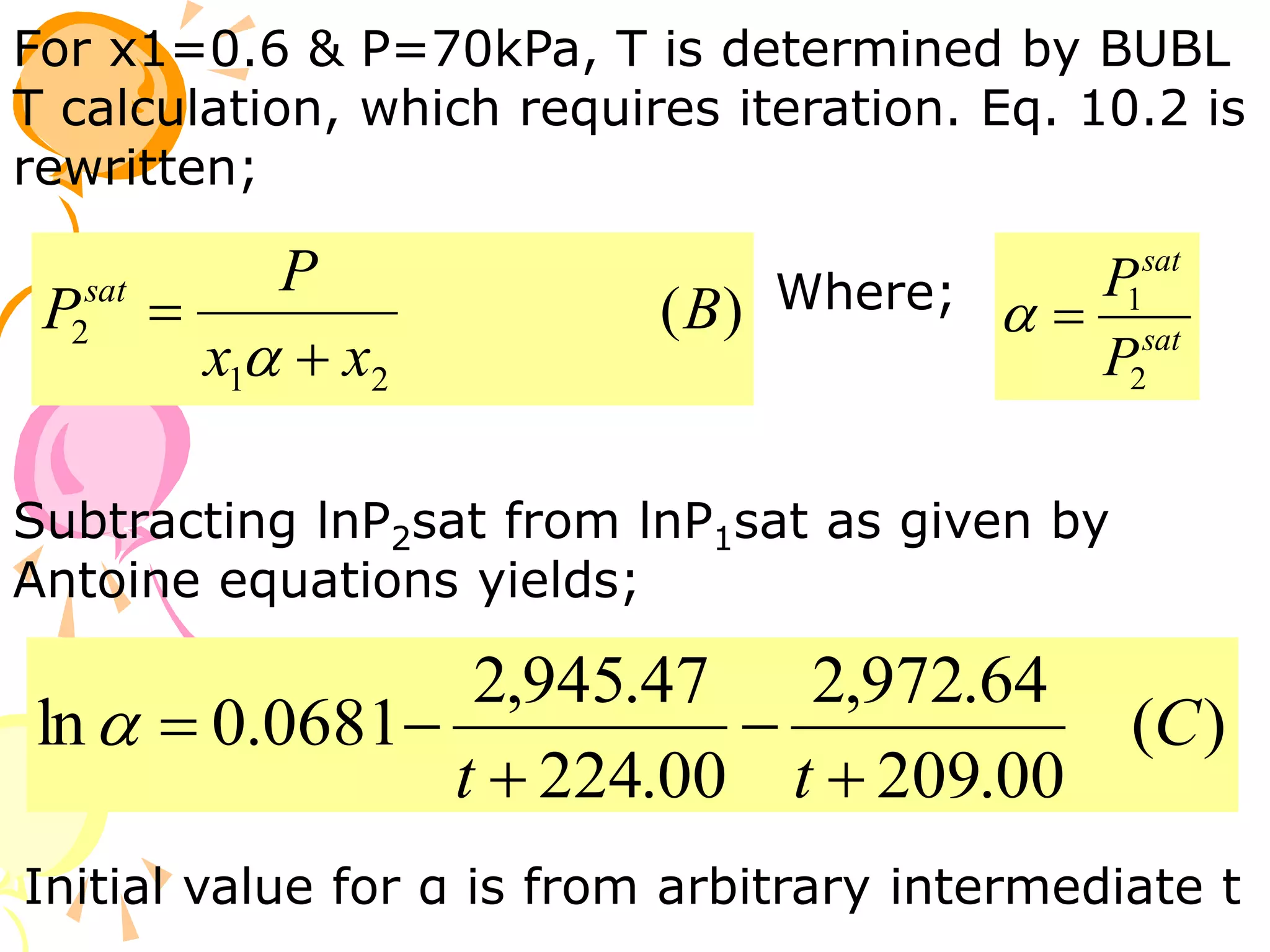
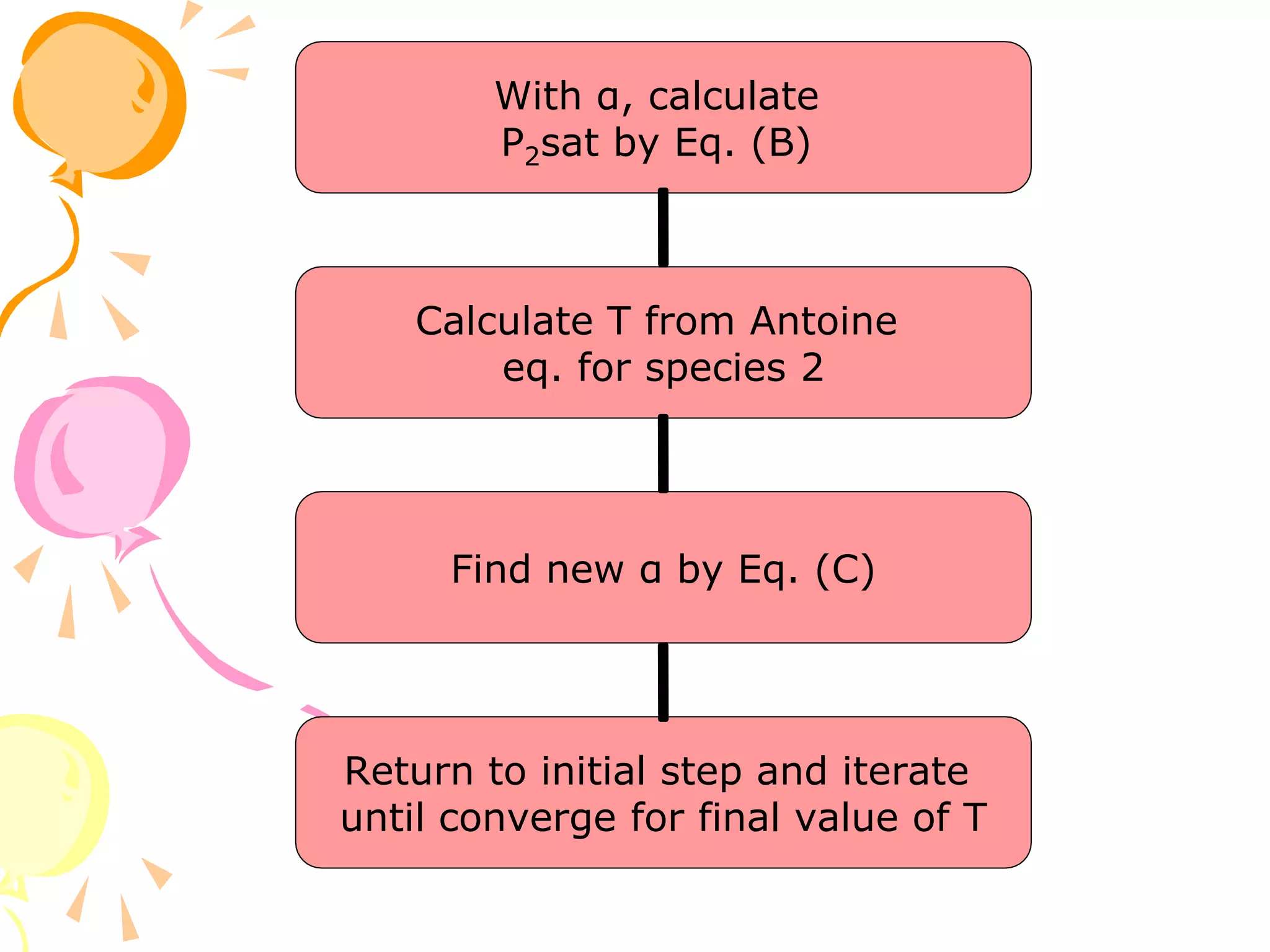
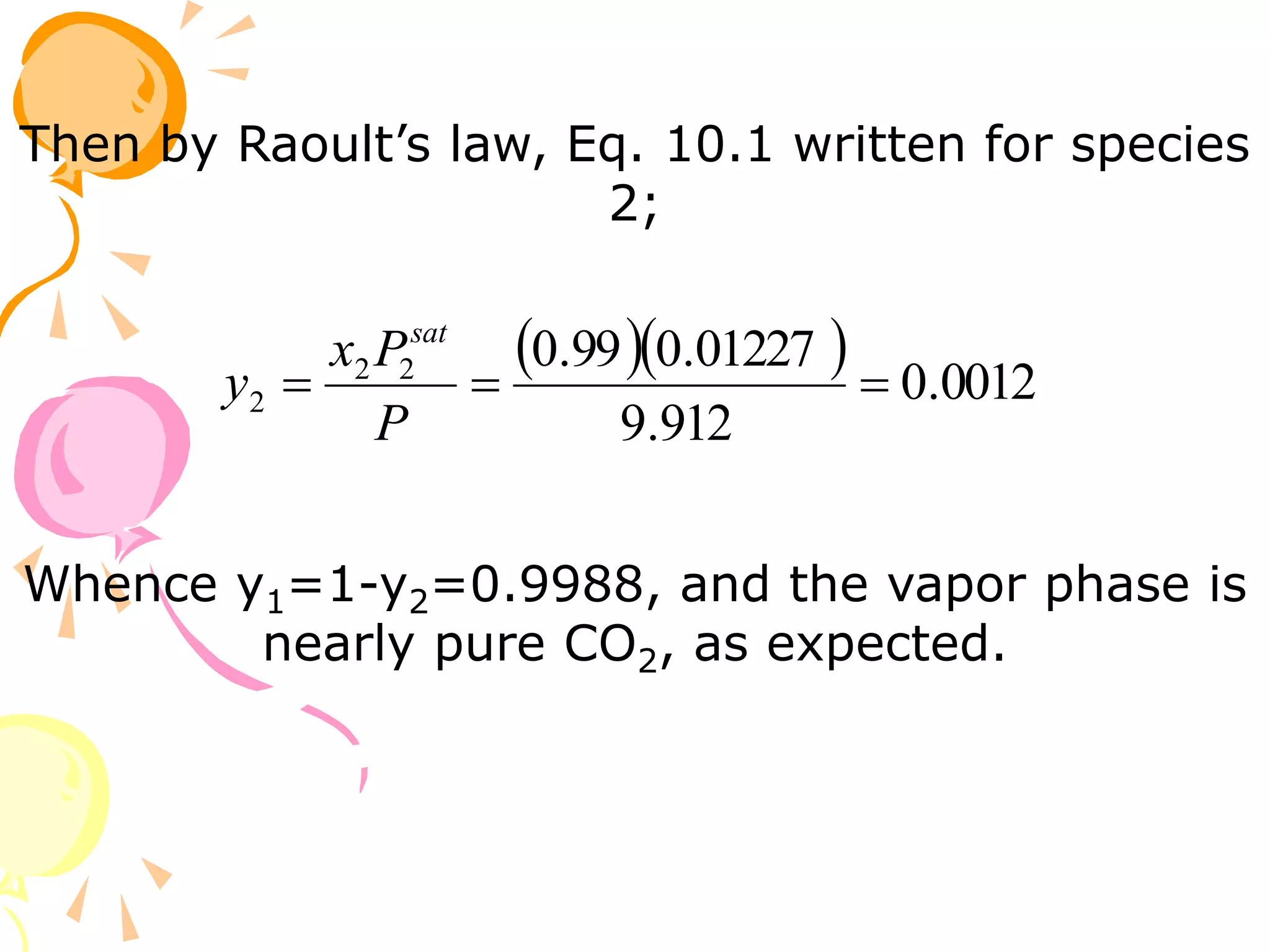
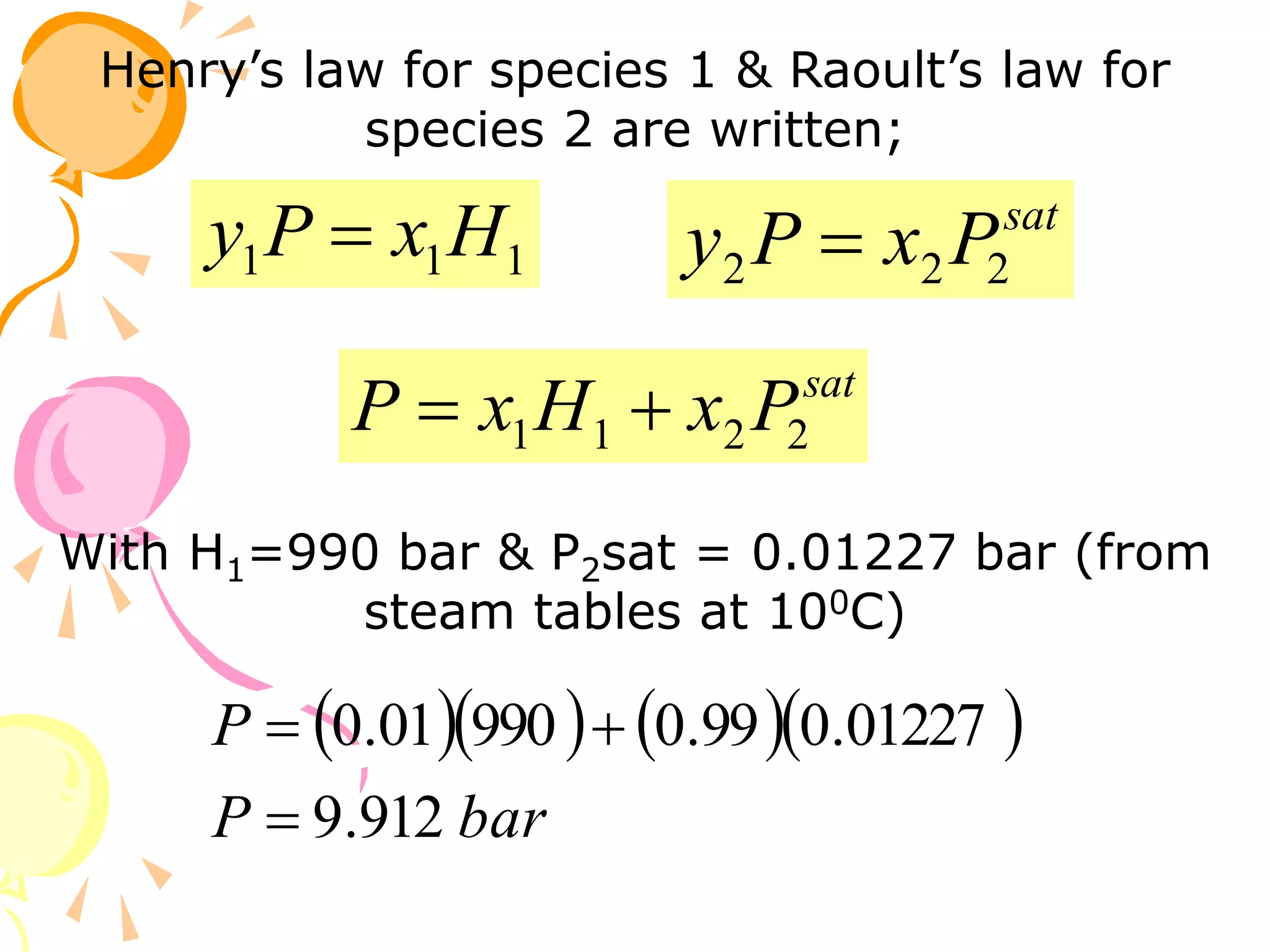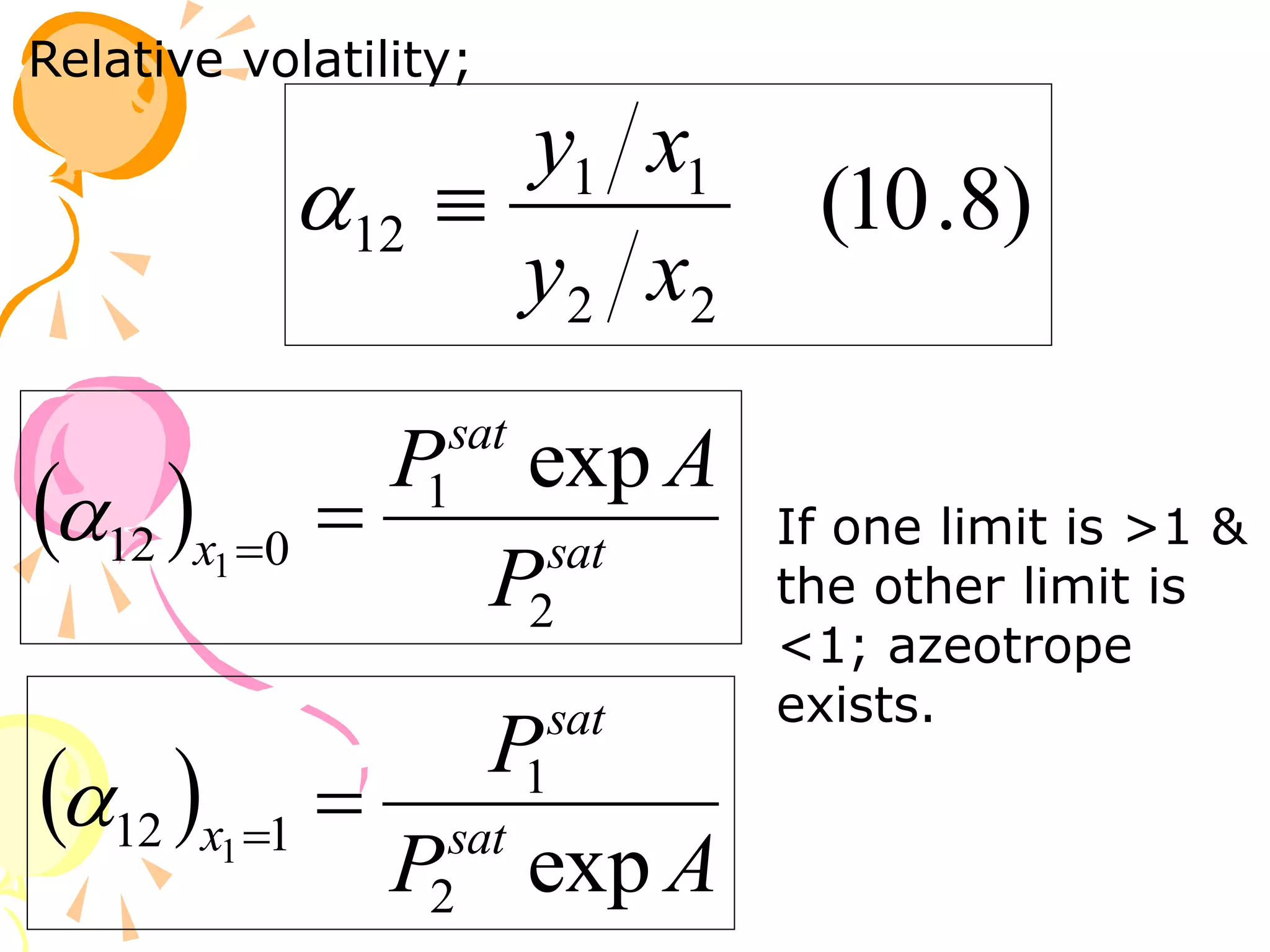1. The document discusses vapor-liquid equilibrium (VLE) and some simple models for calculating VLE, including Raoult's law and Henry's law.
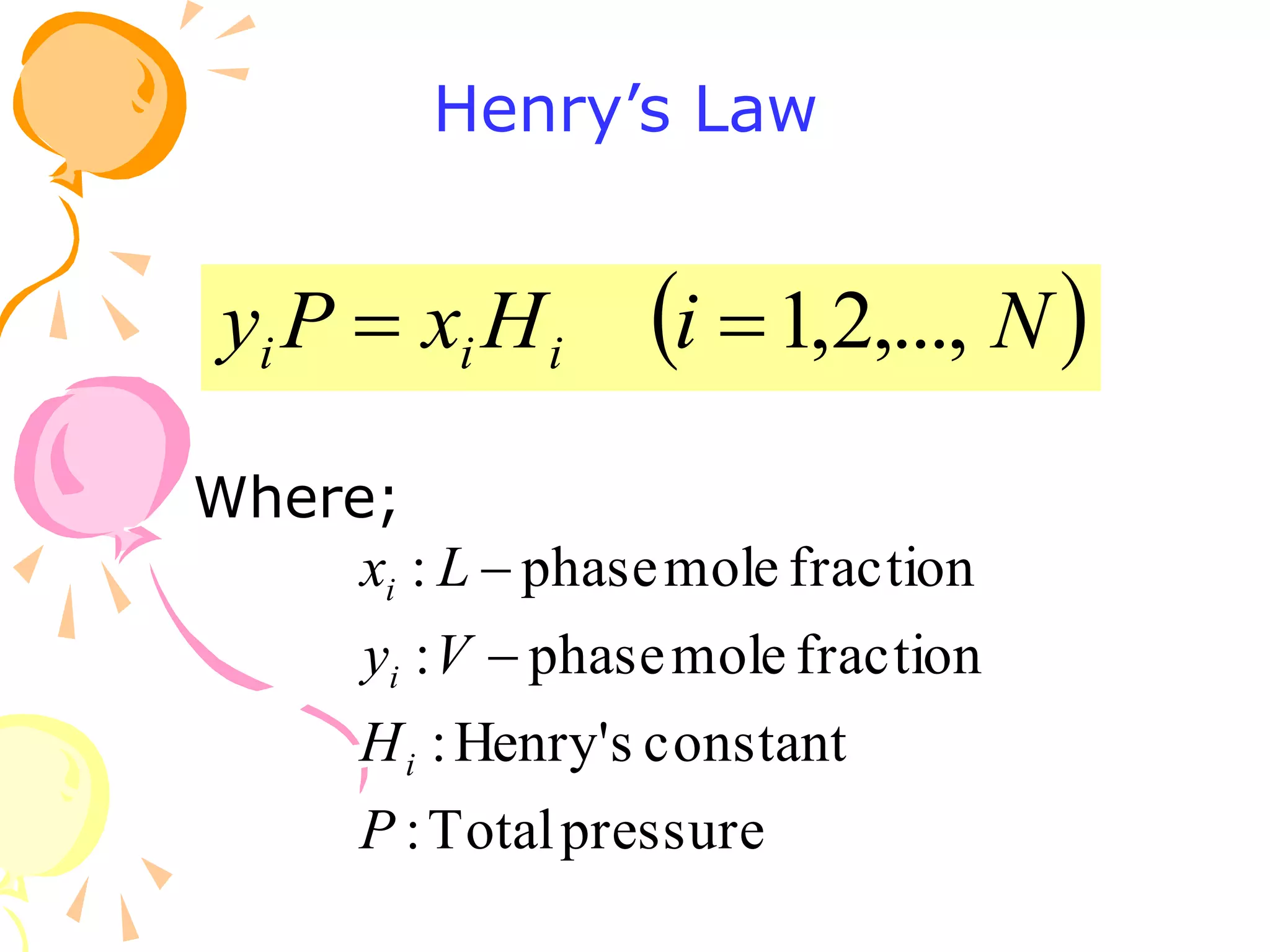
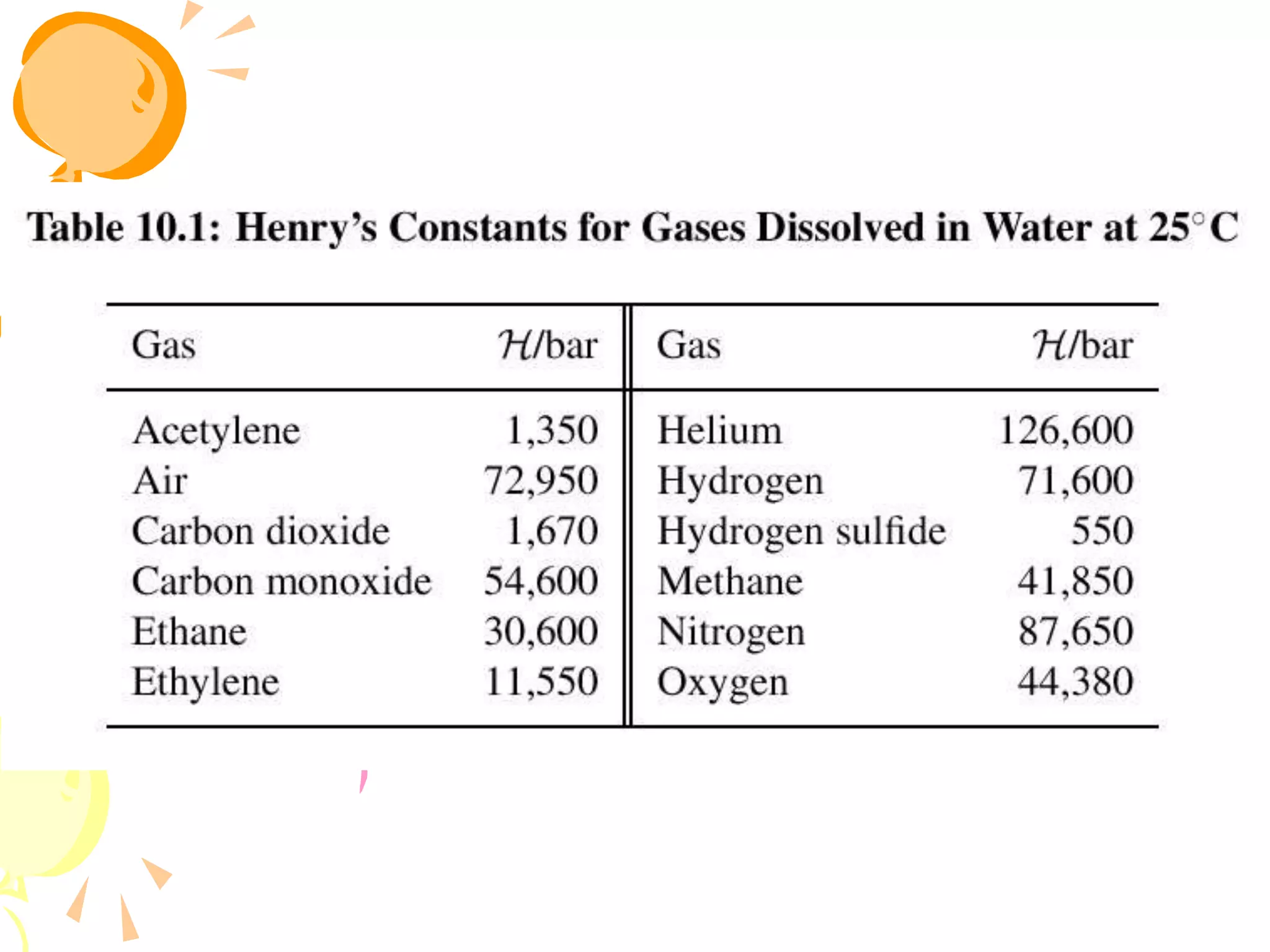
2. Raoult's law assumes an ideal gas in the vapor phase and an ideal solution in the liquid phase. Henry's law is applicable for very dilute solutions and low pressures where the vapor can be treated as an ideal gas.
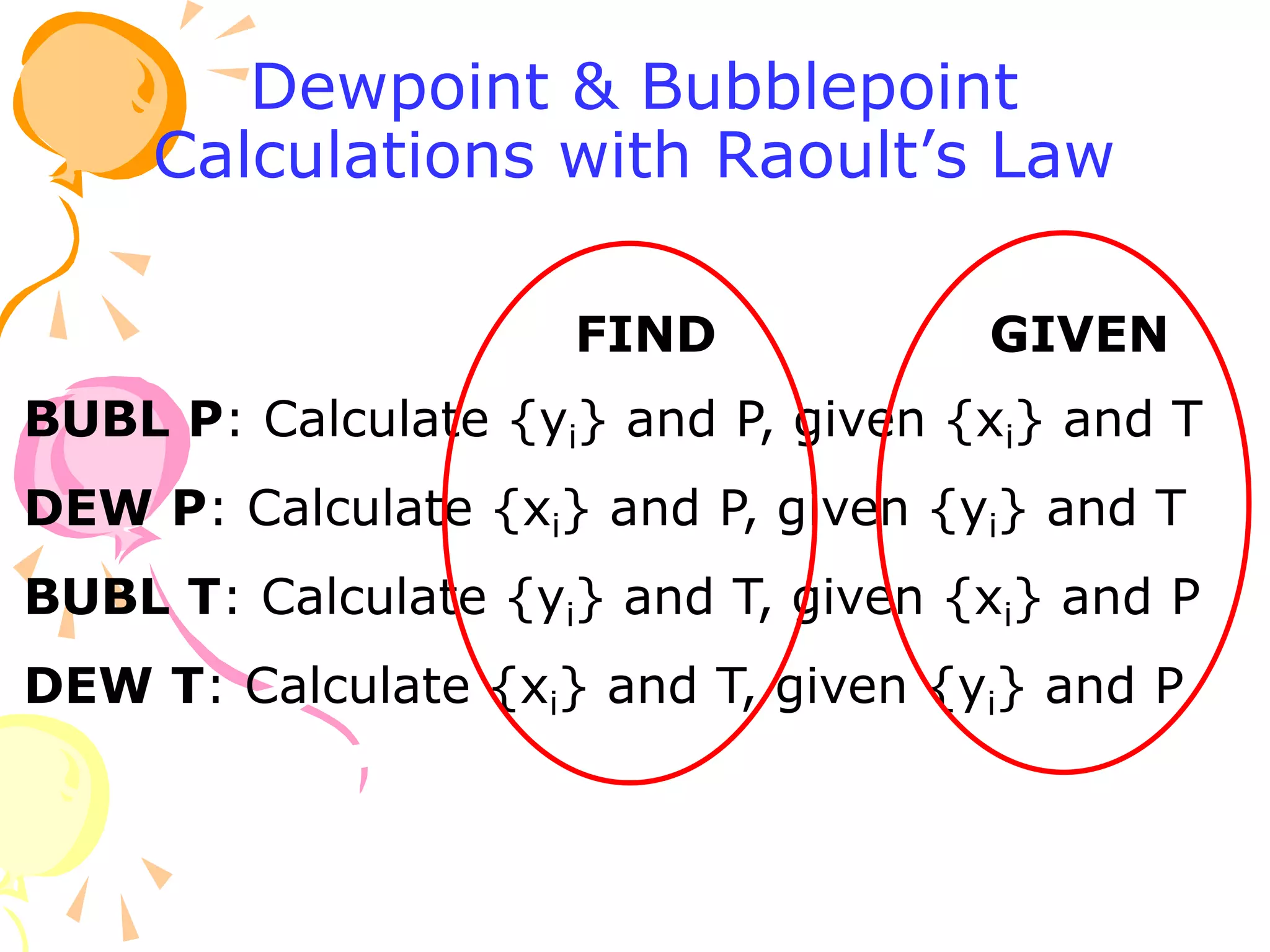
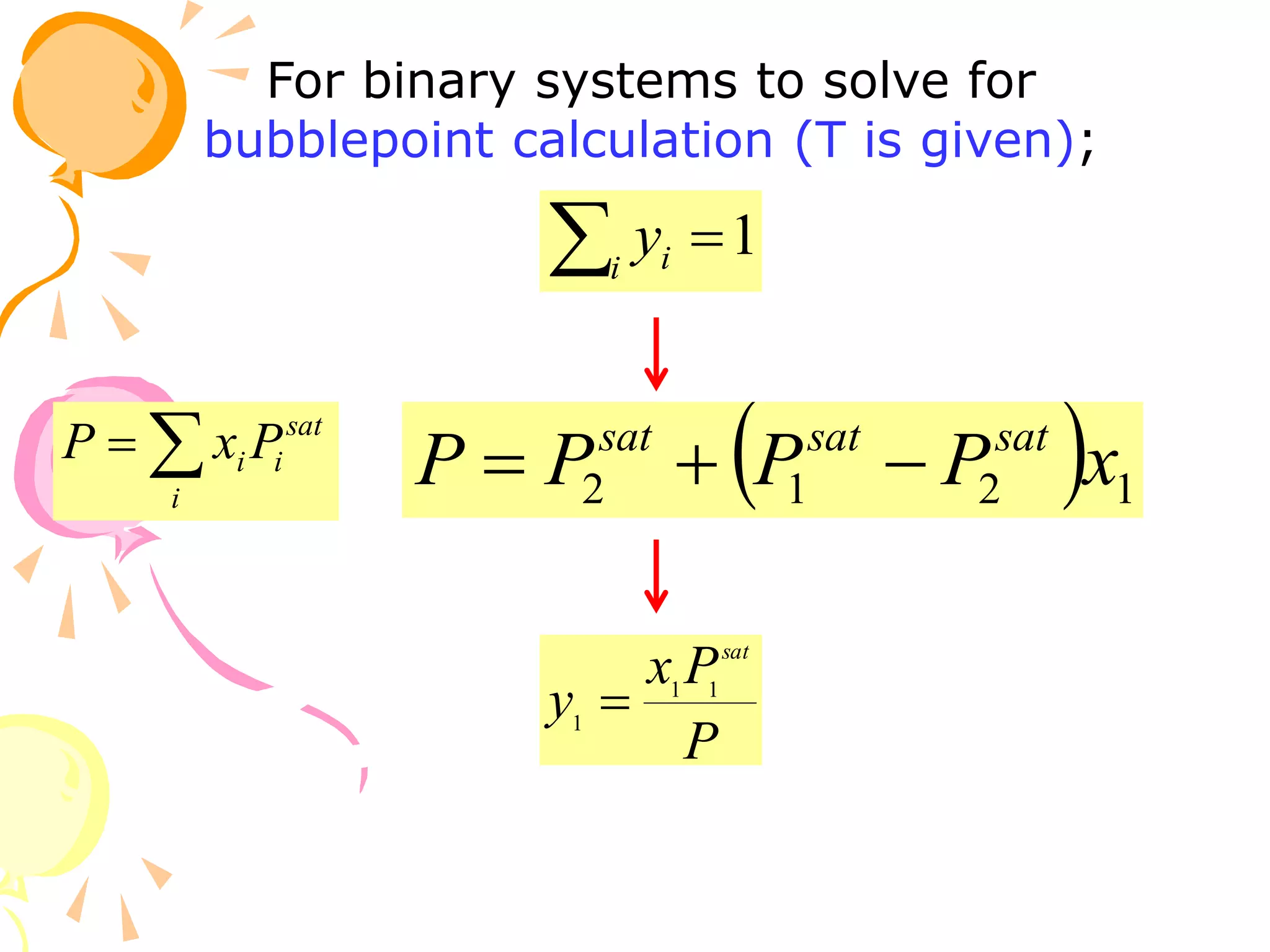
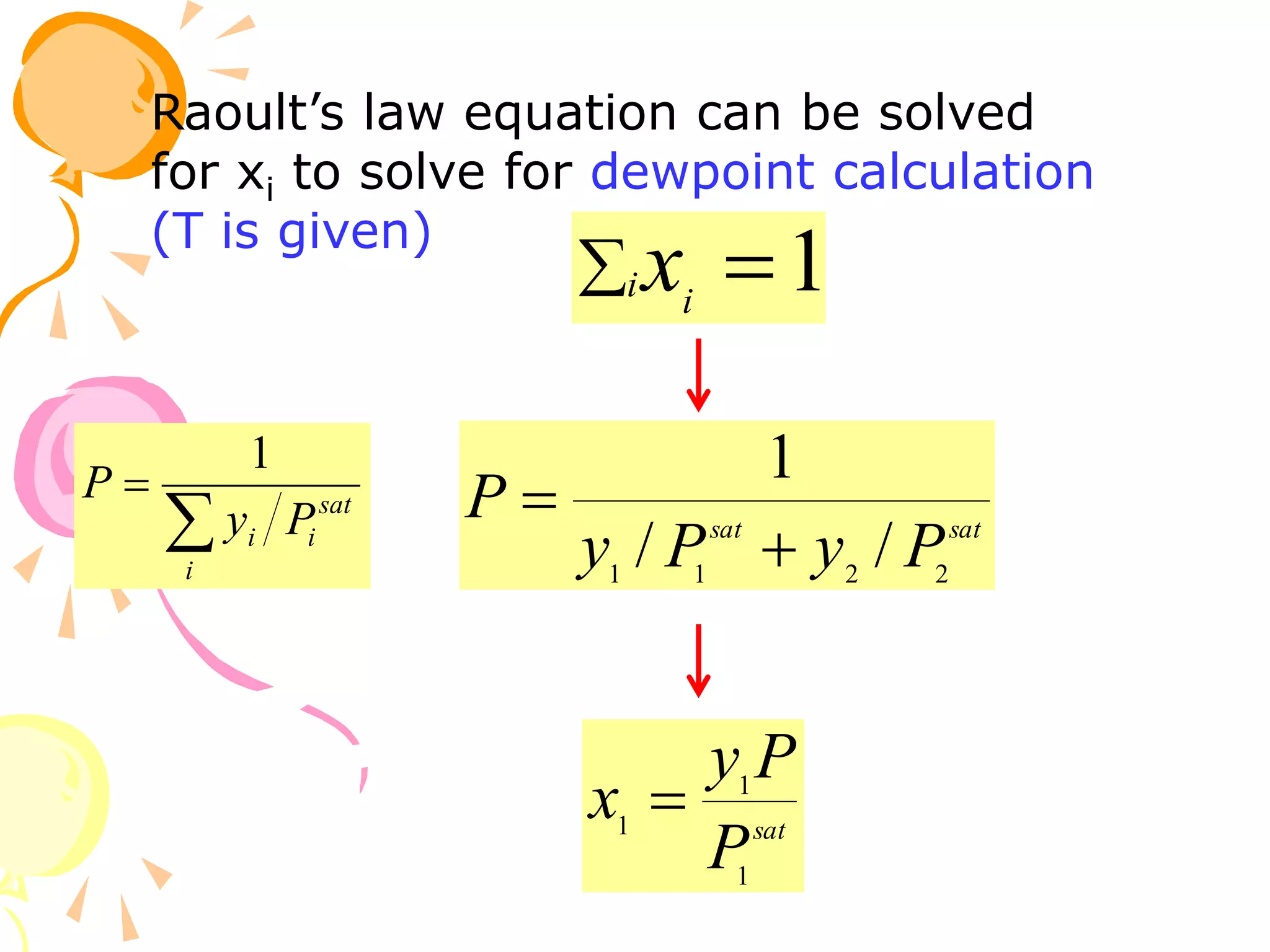
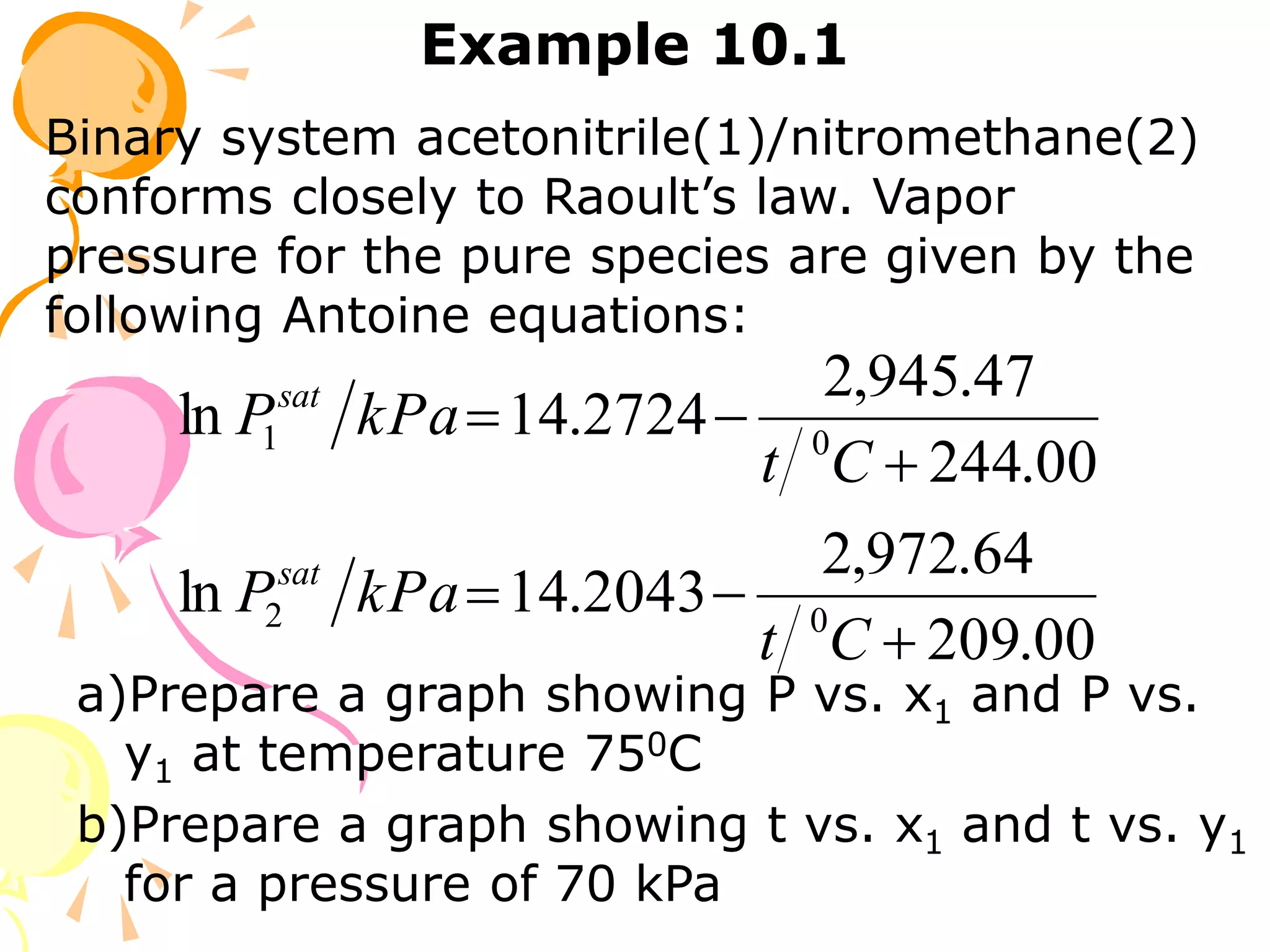
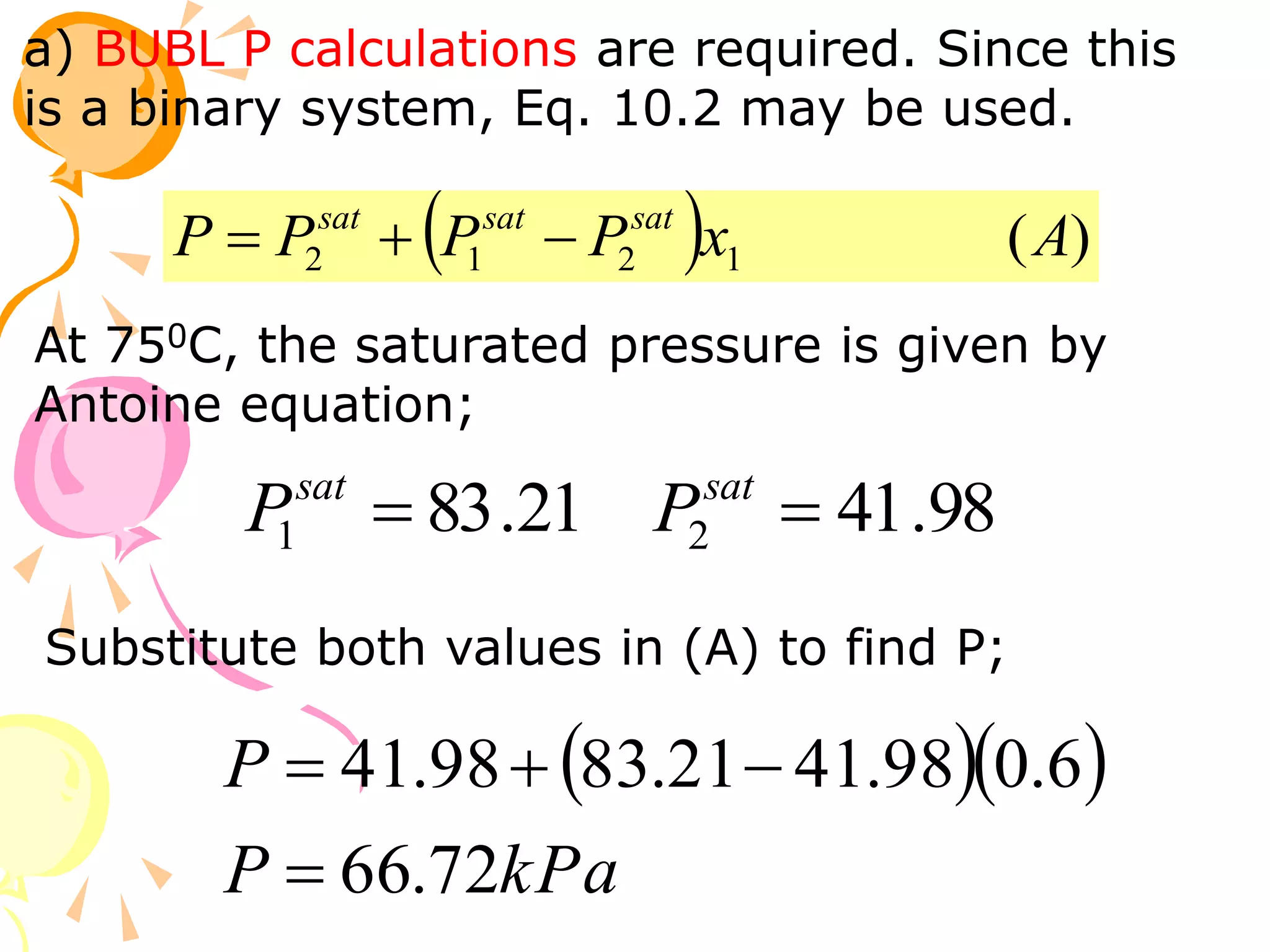
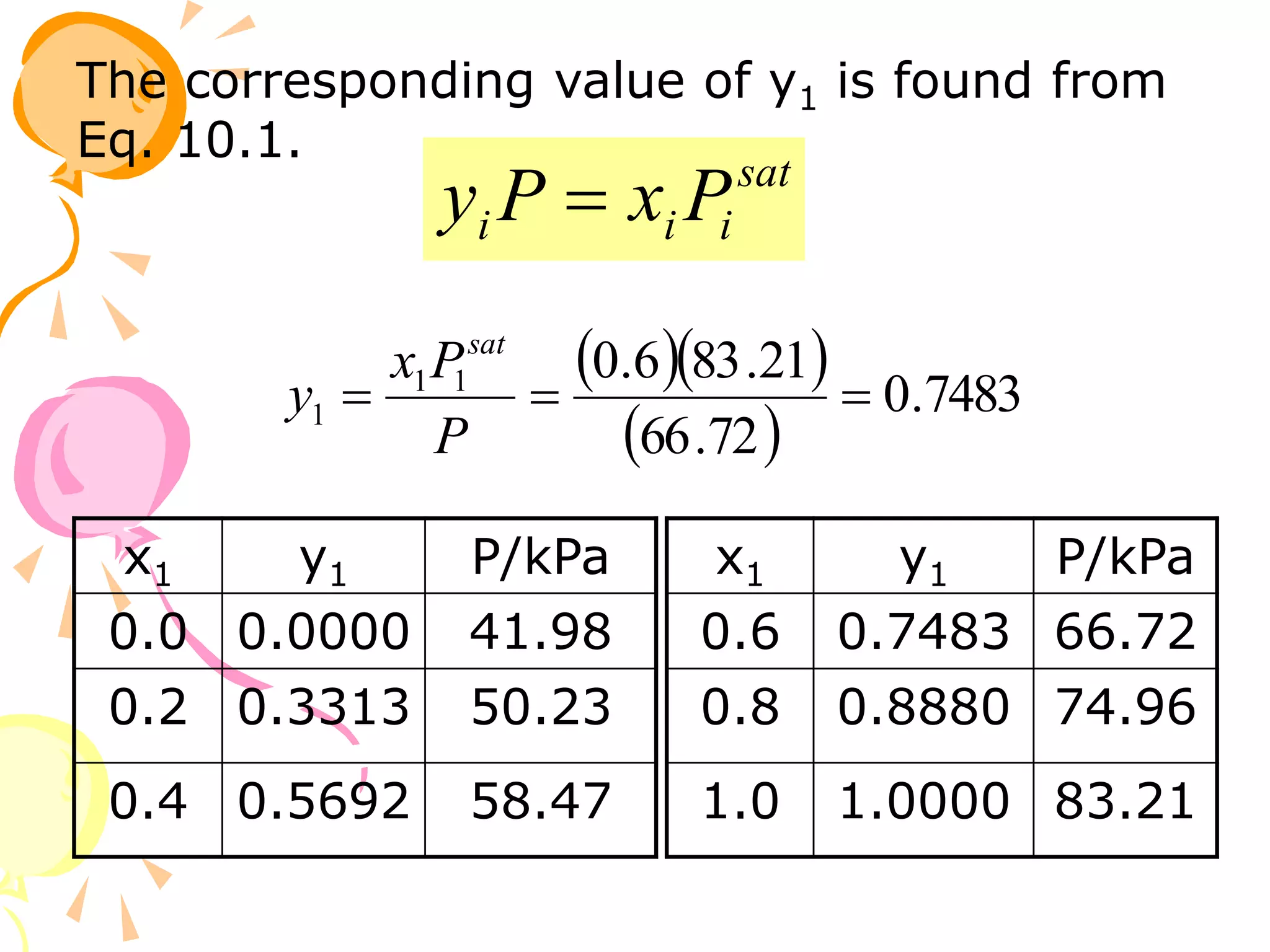
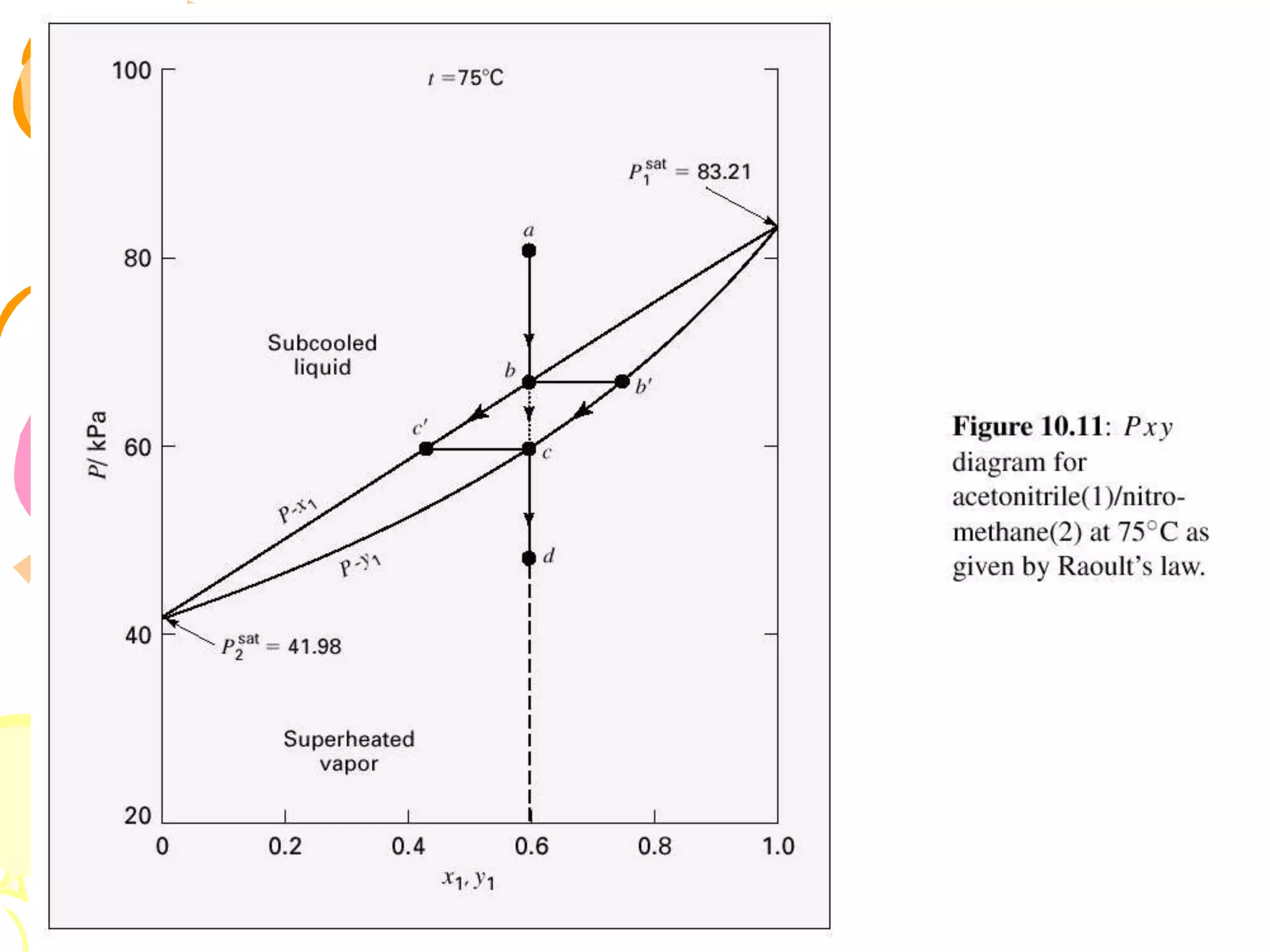
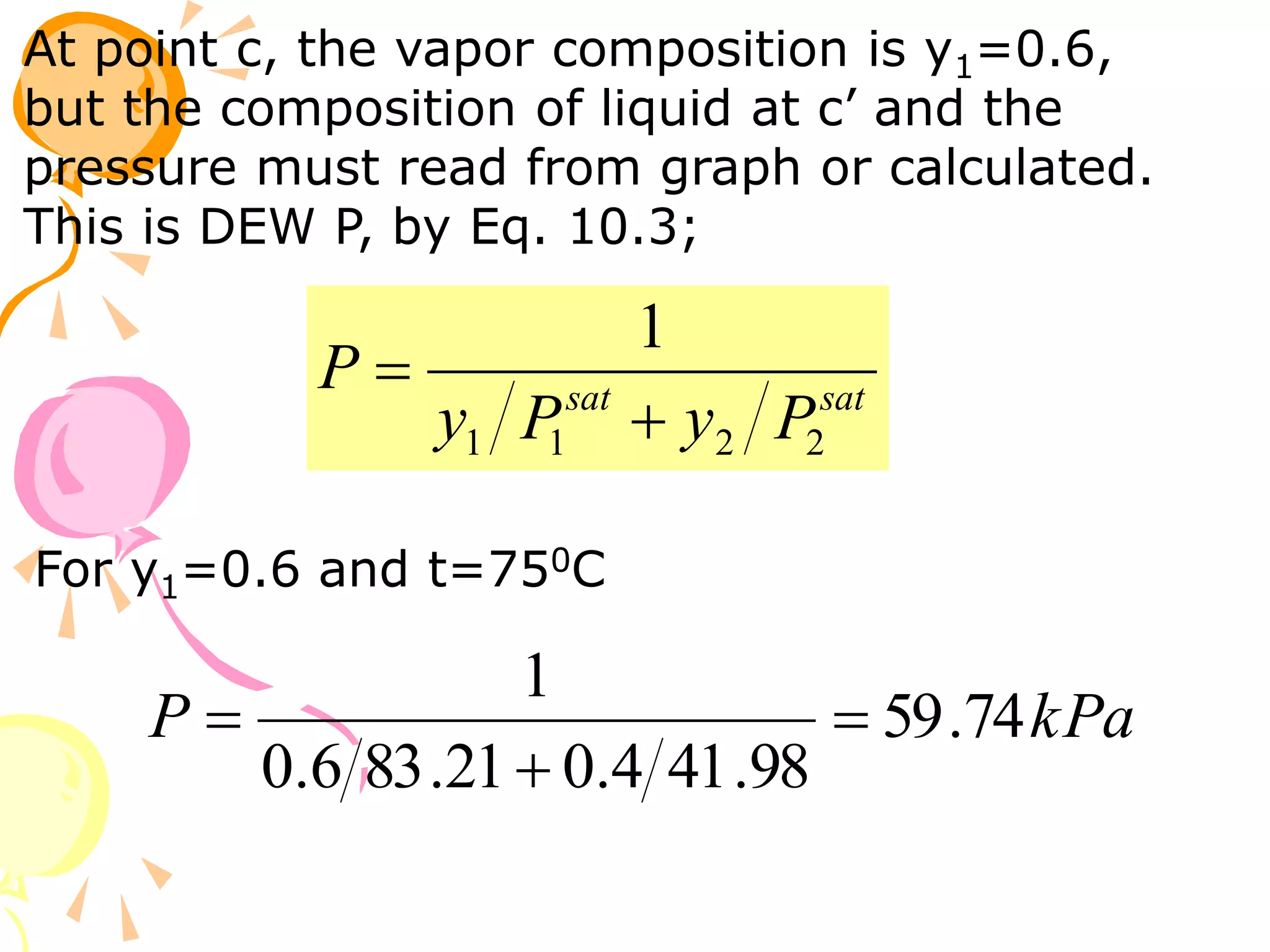
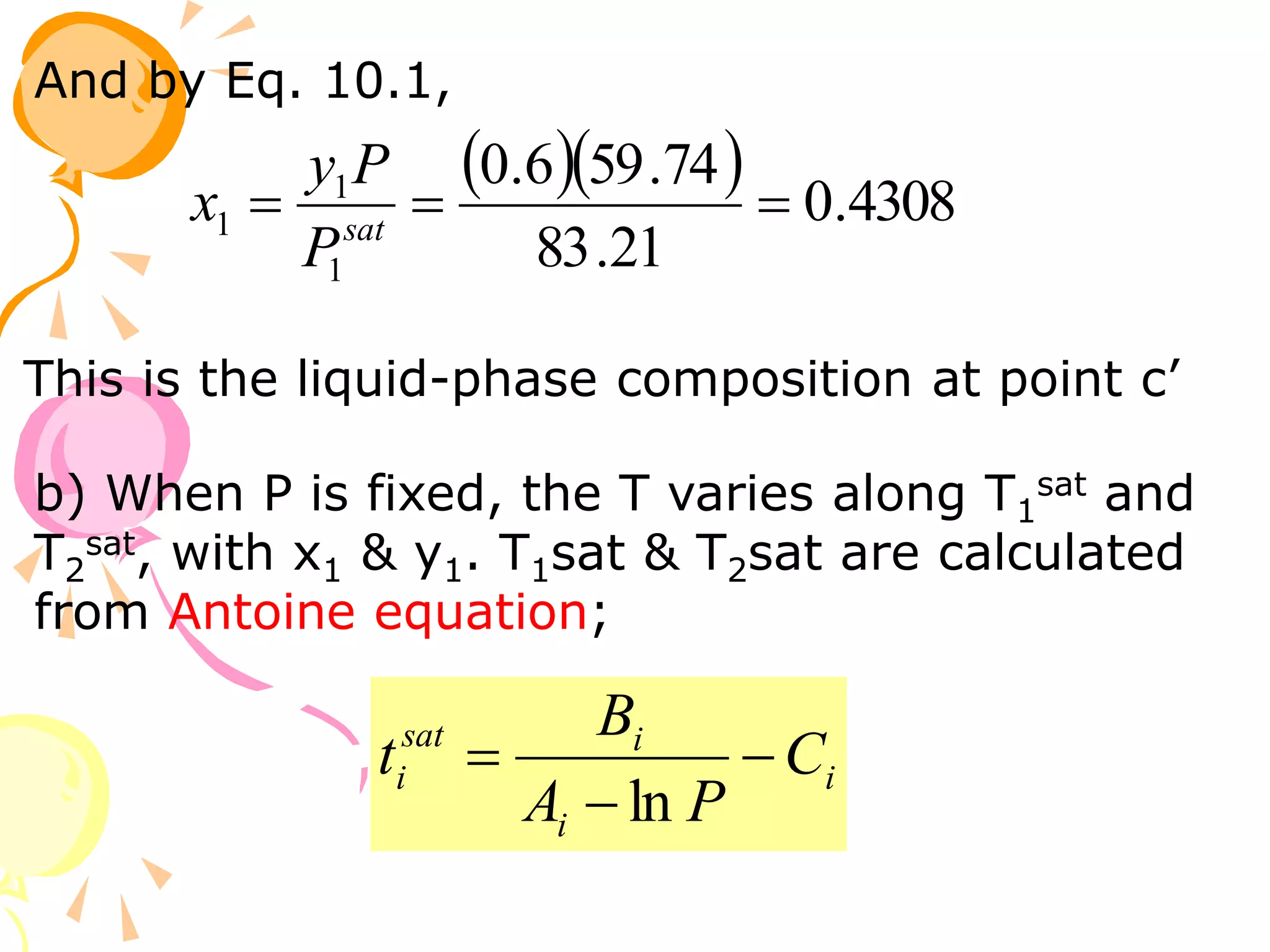
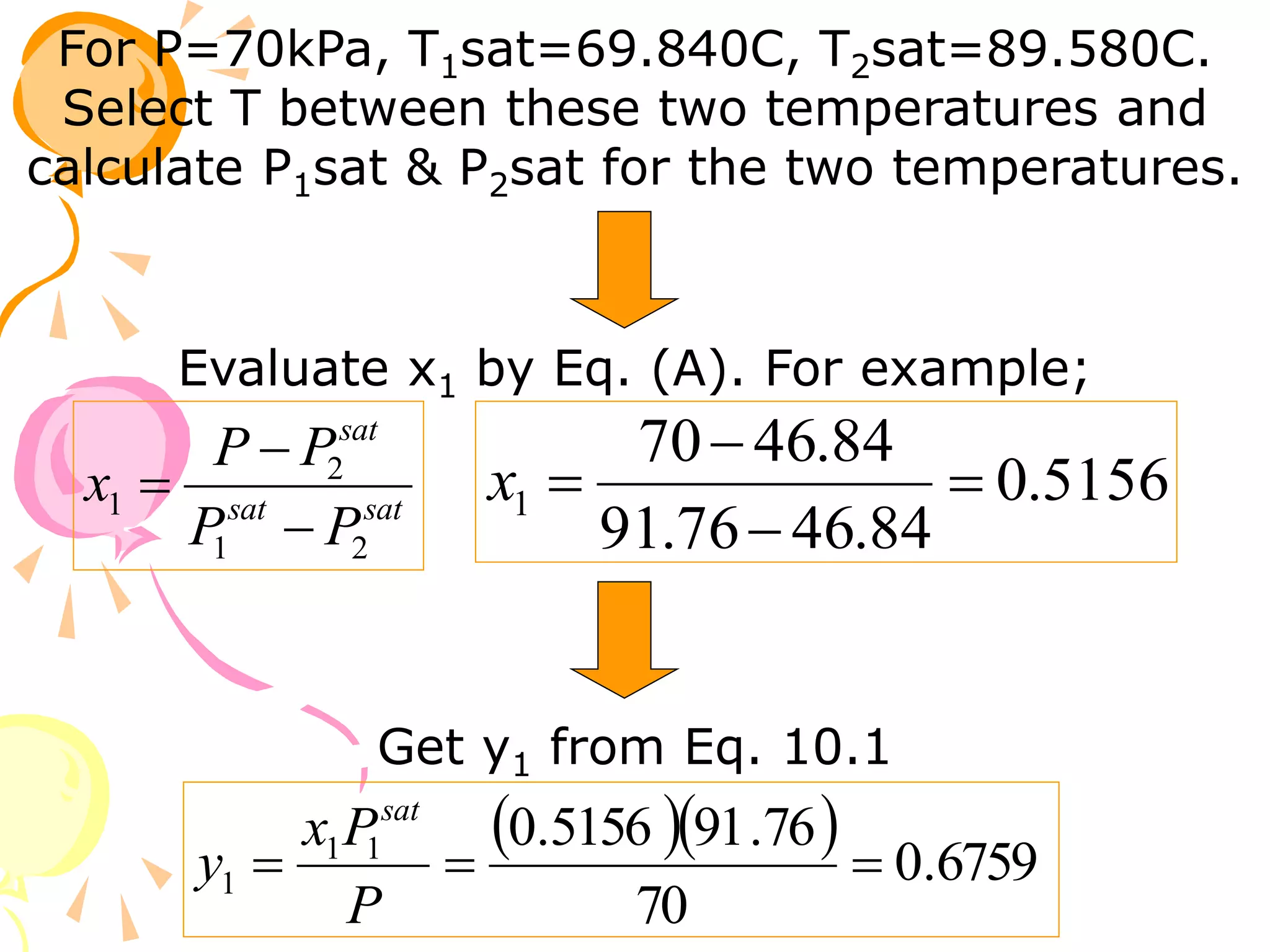
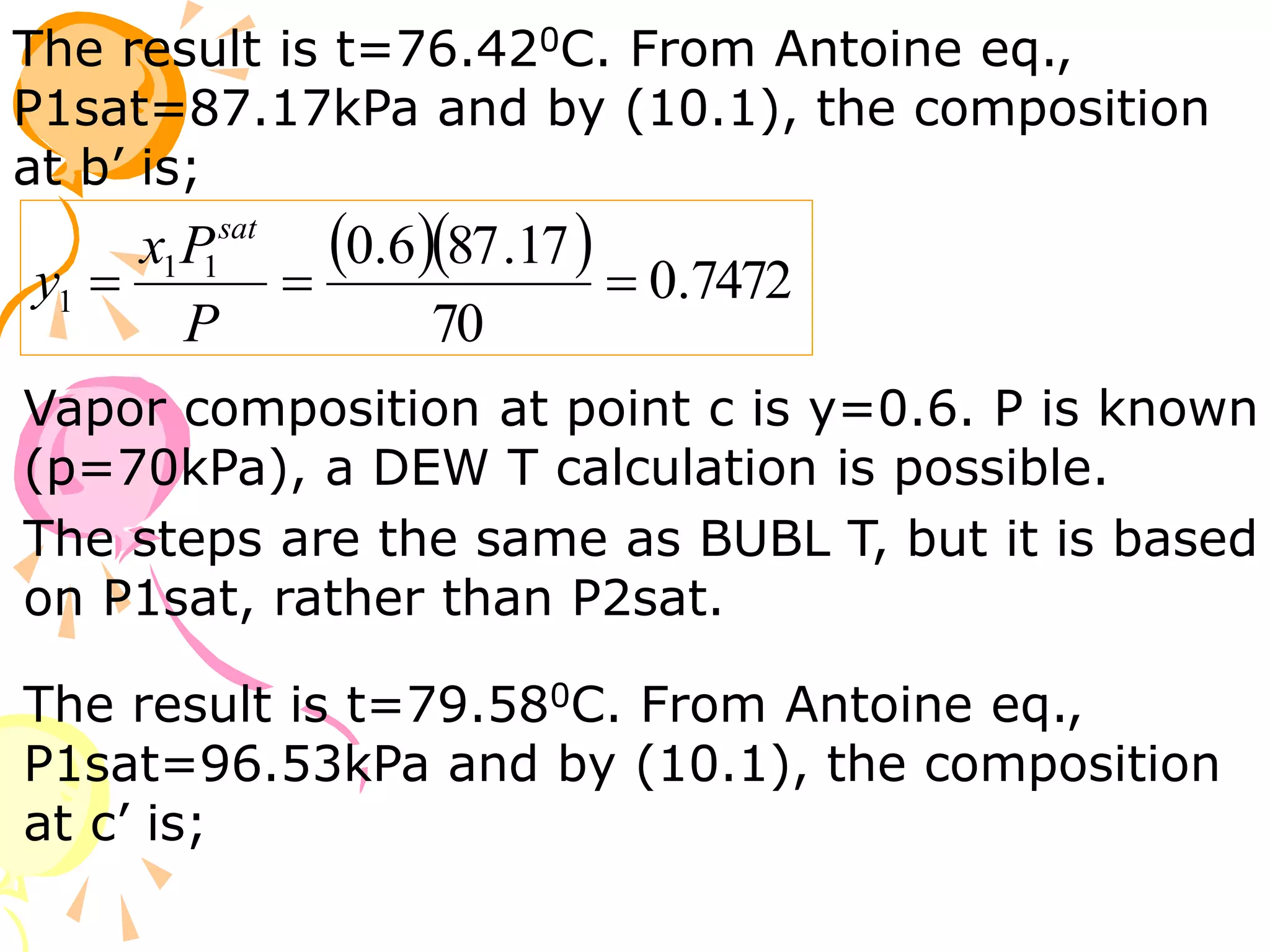
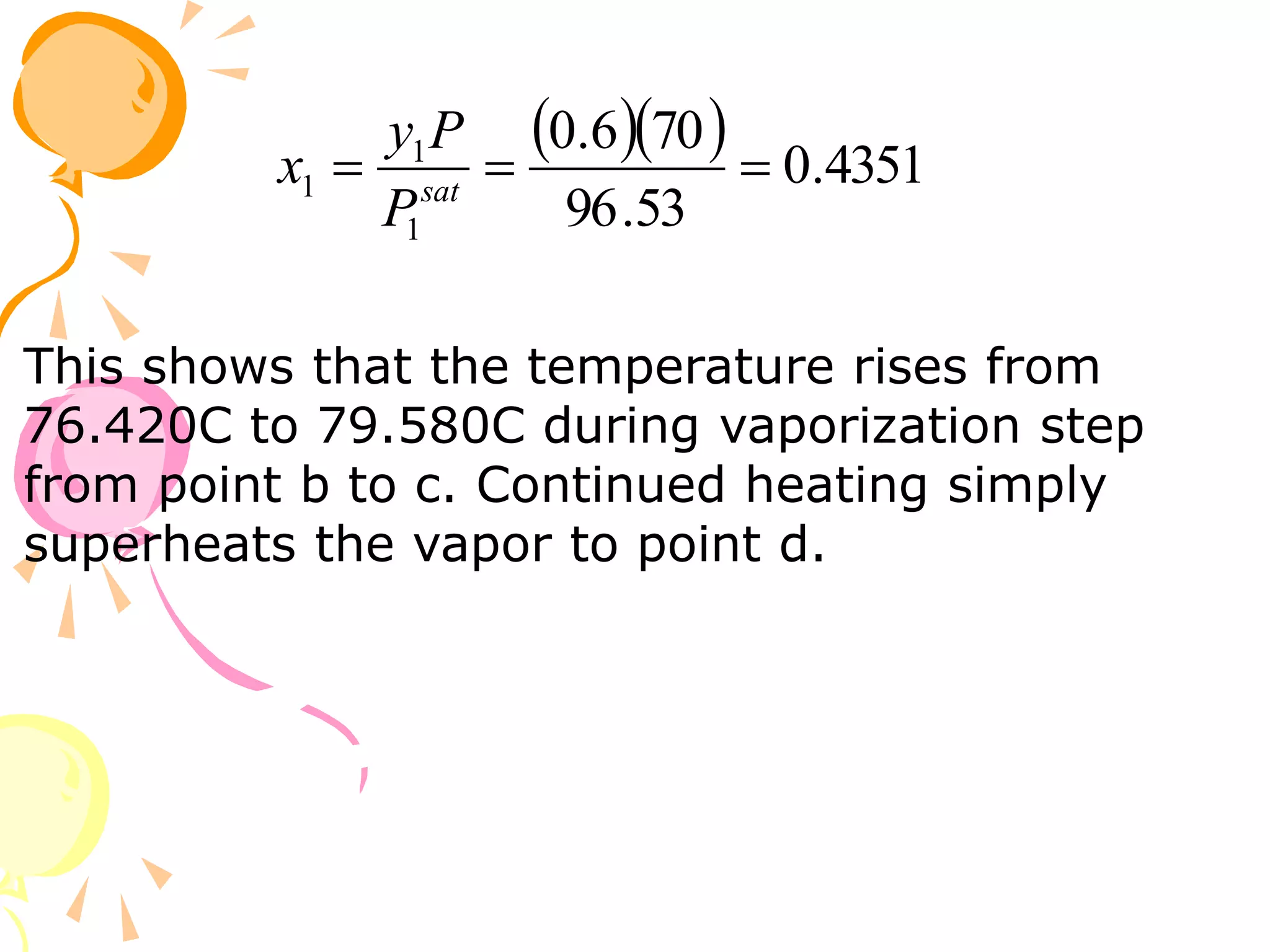
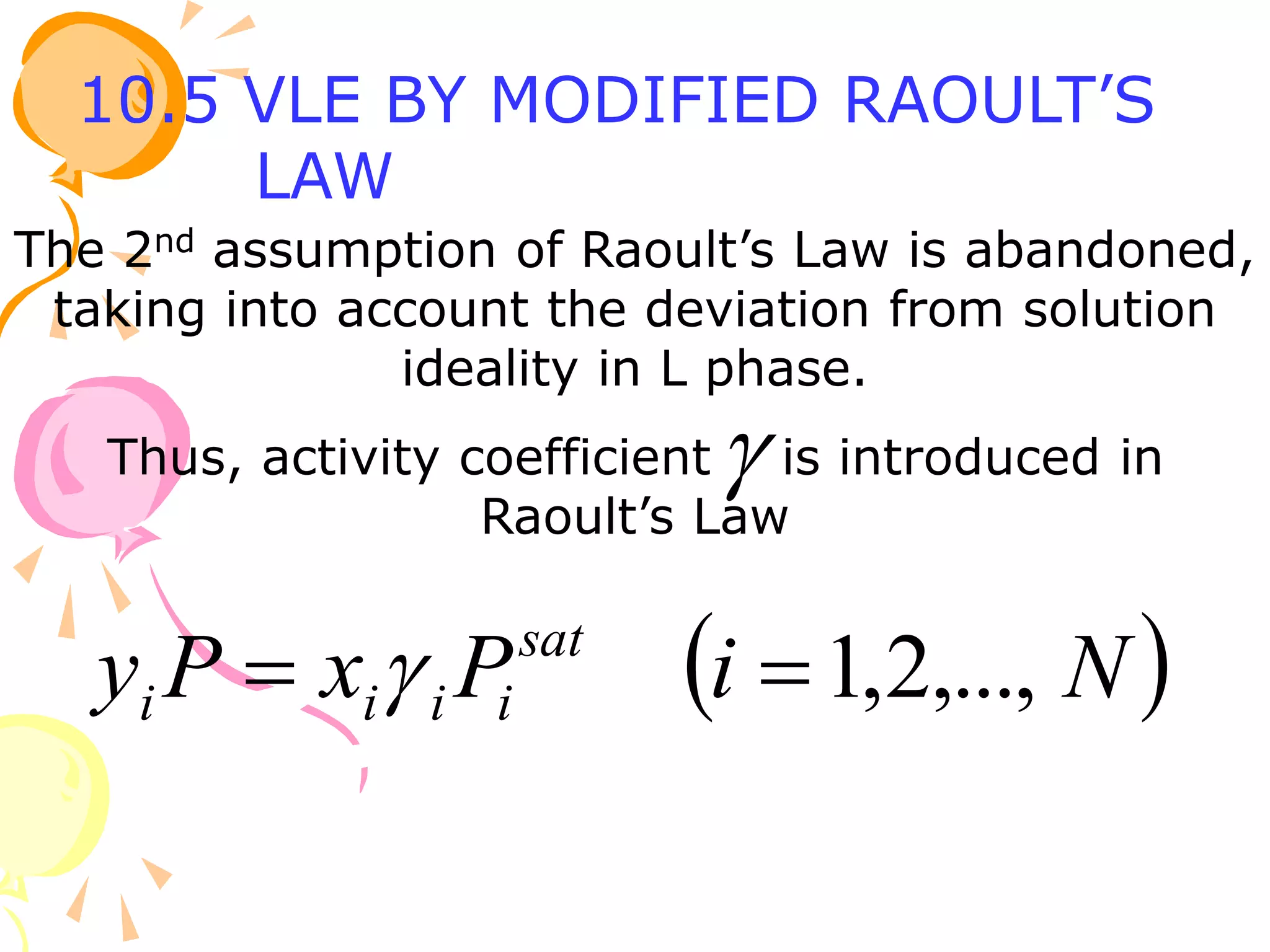
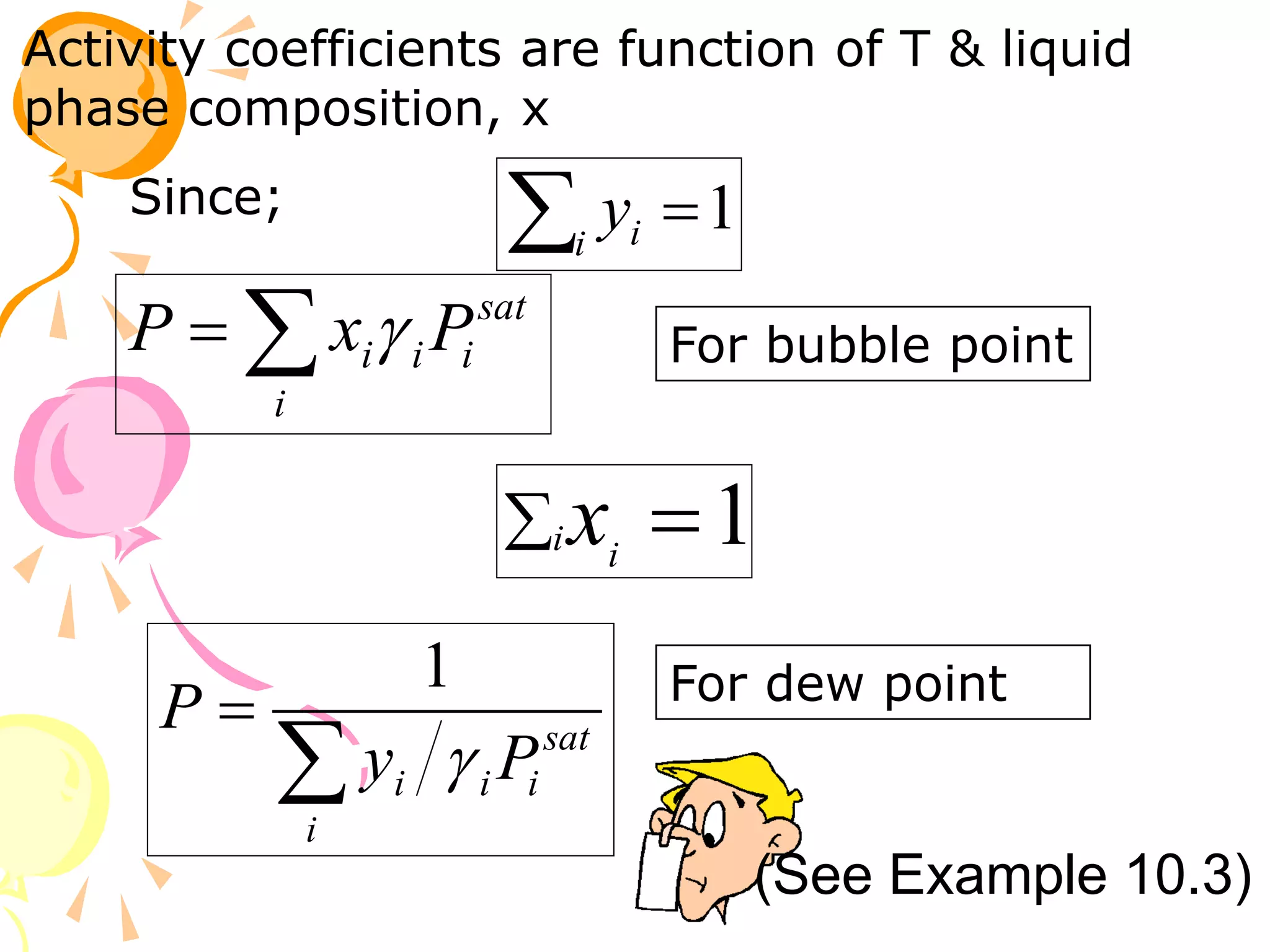
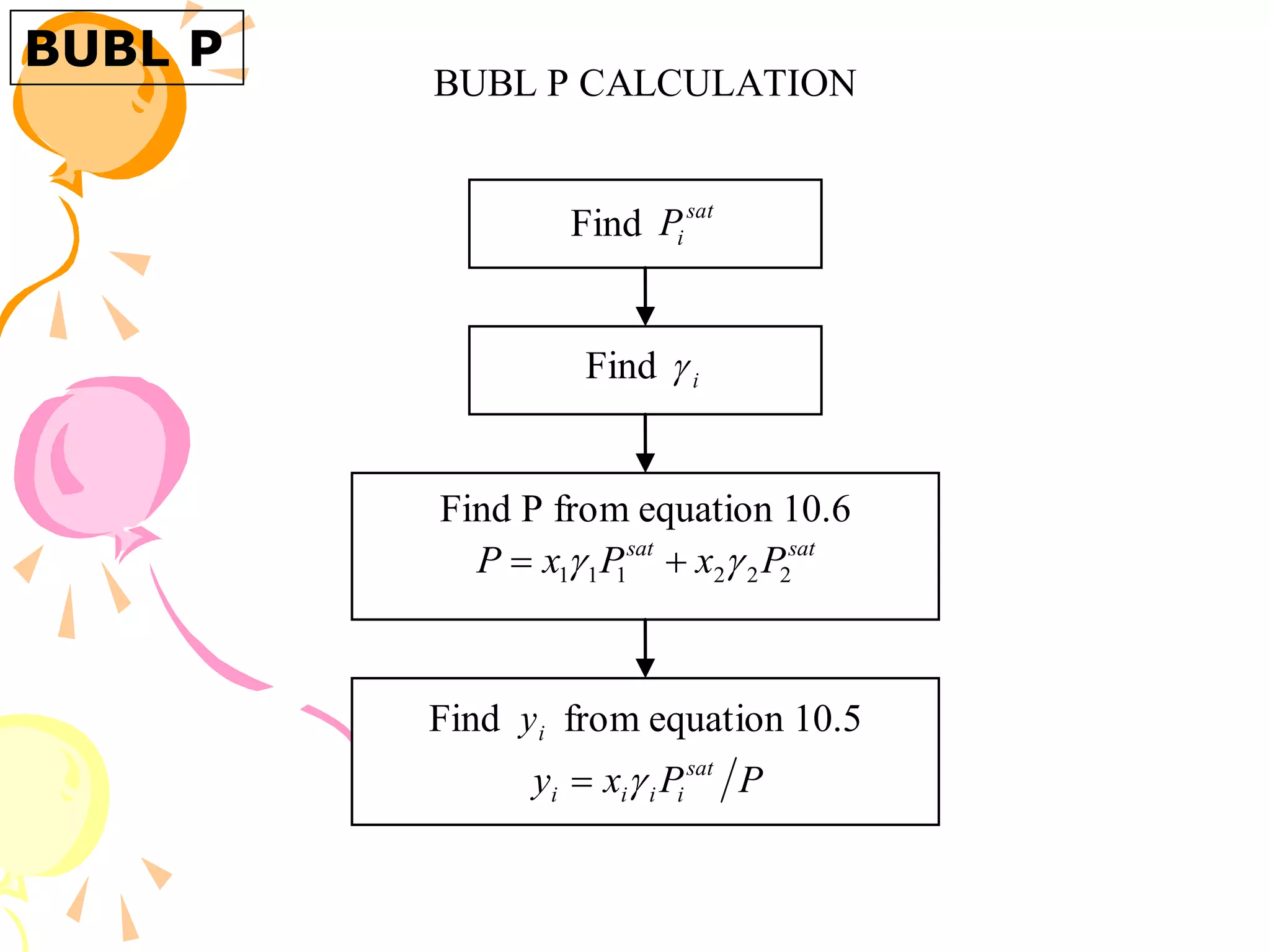
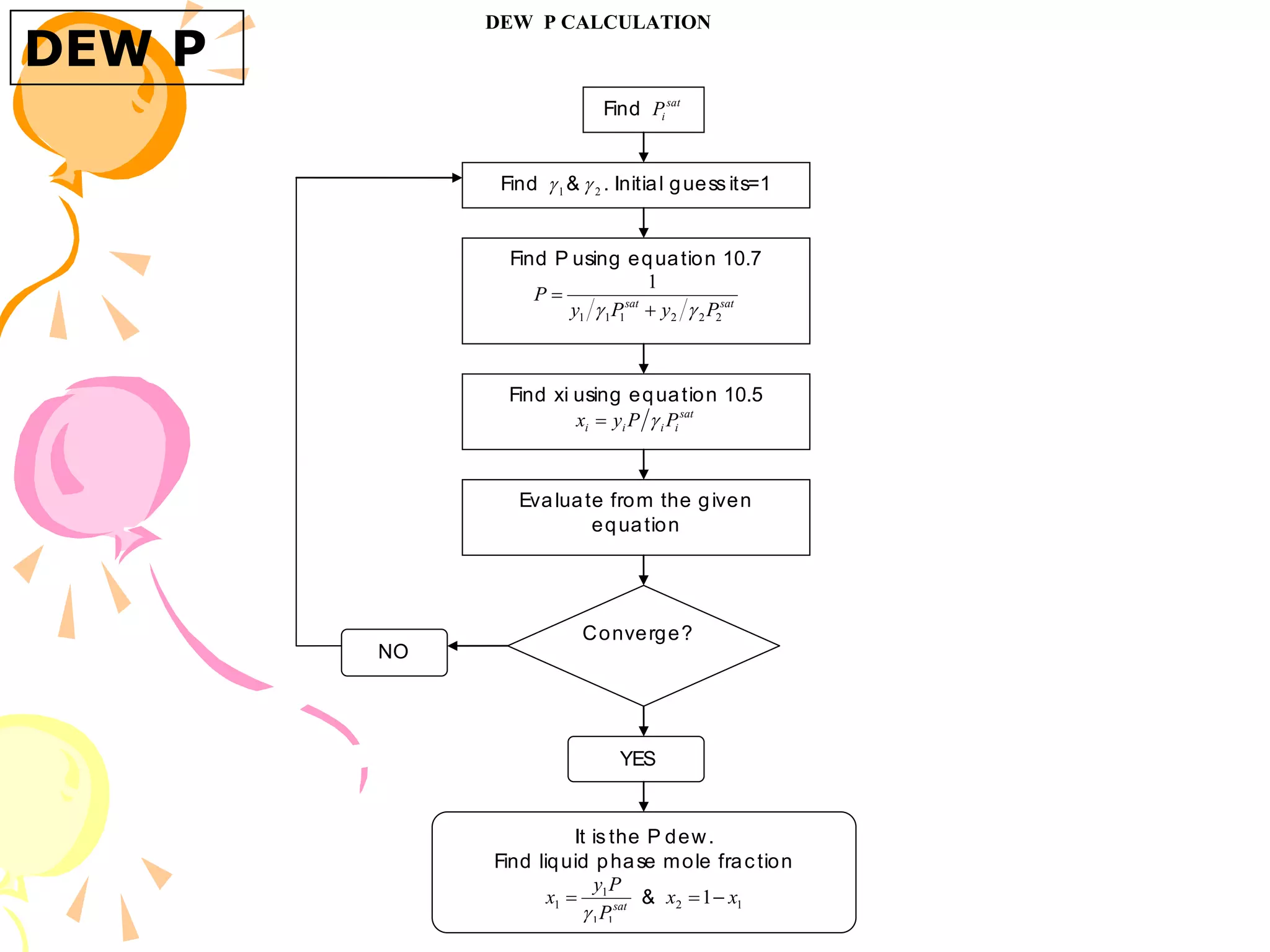
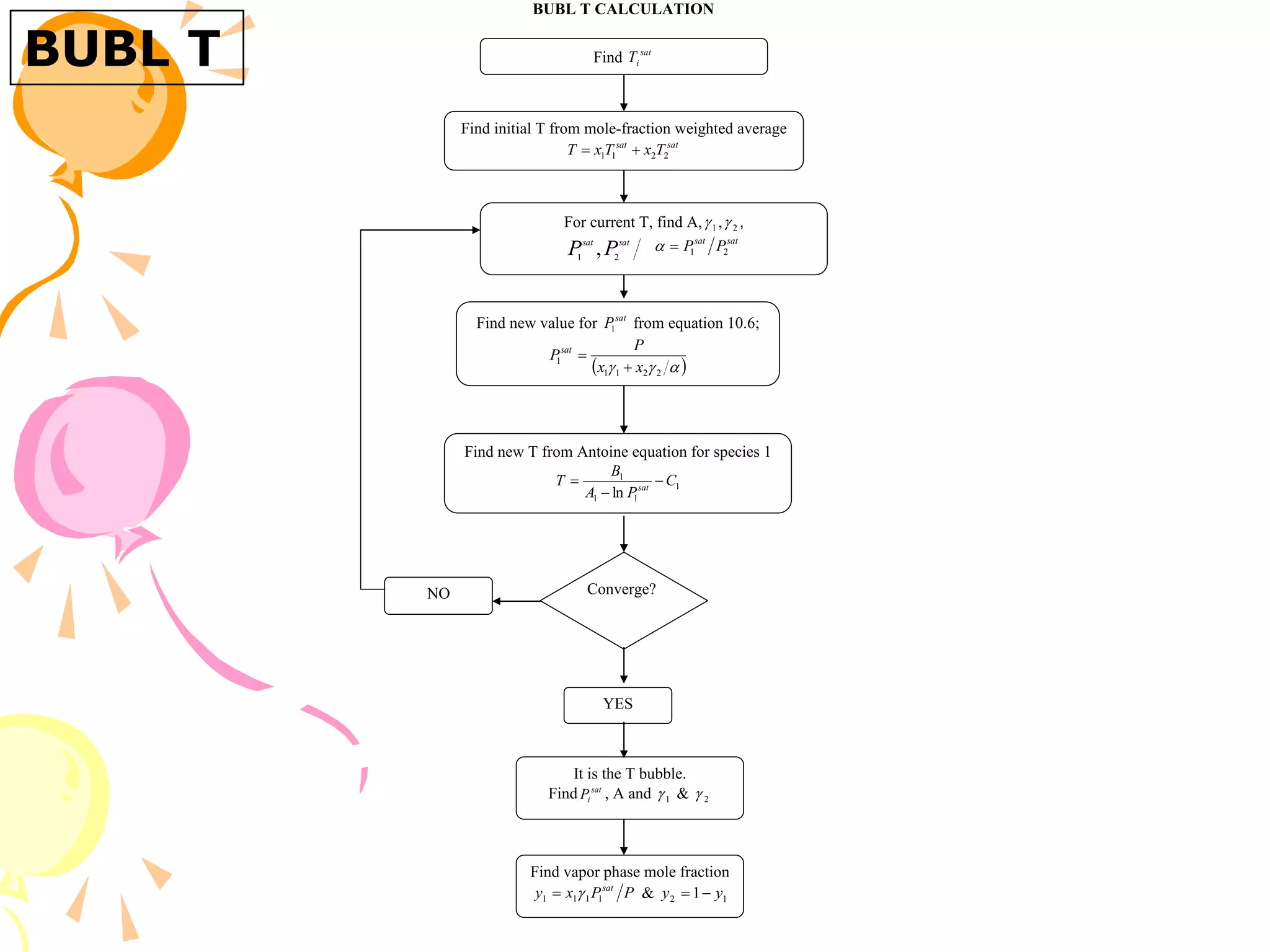
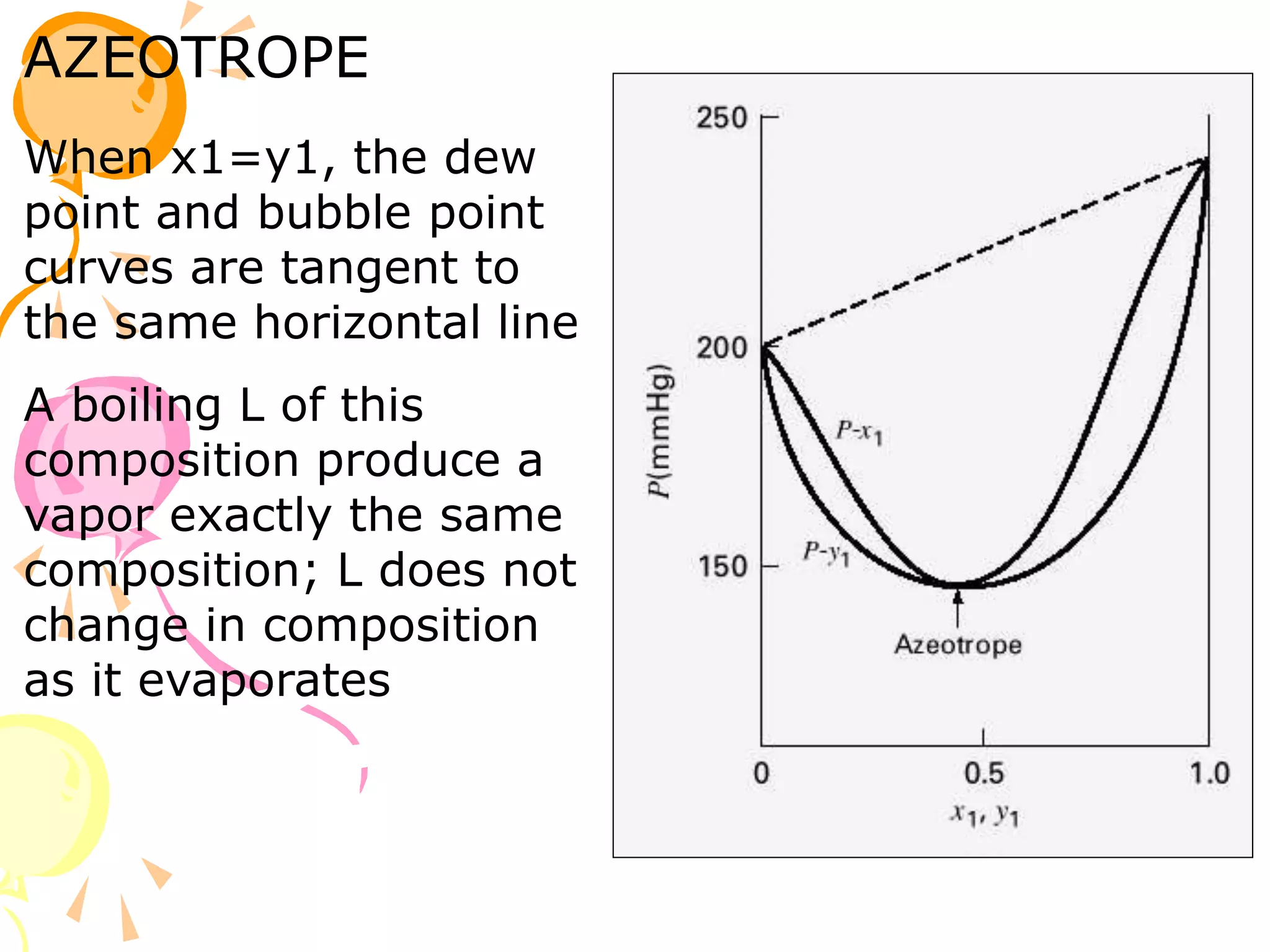
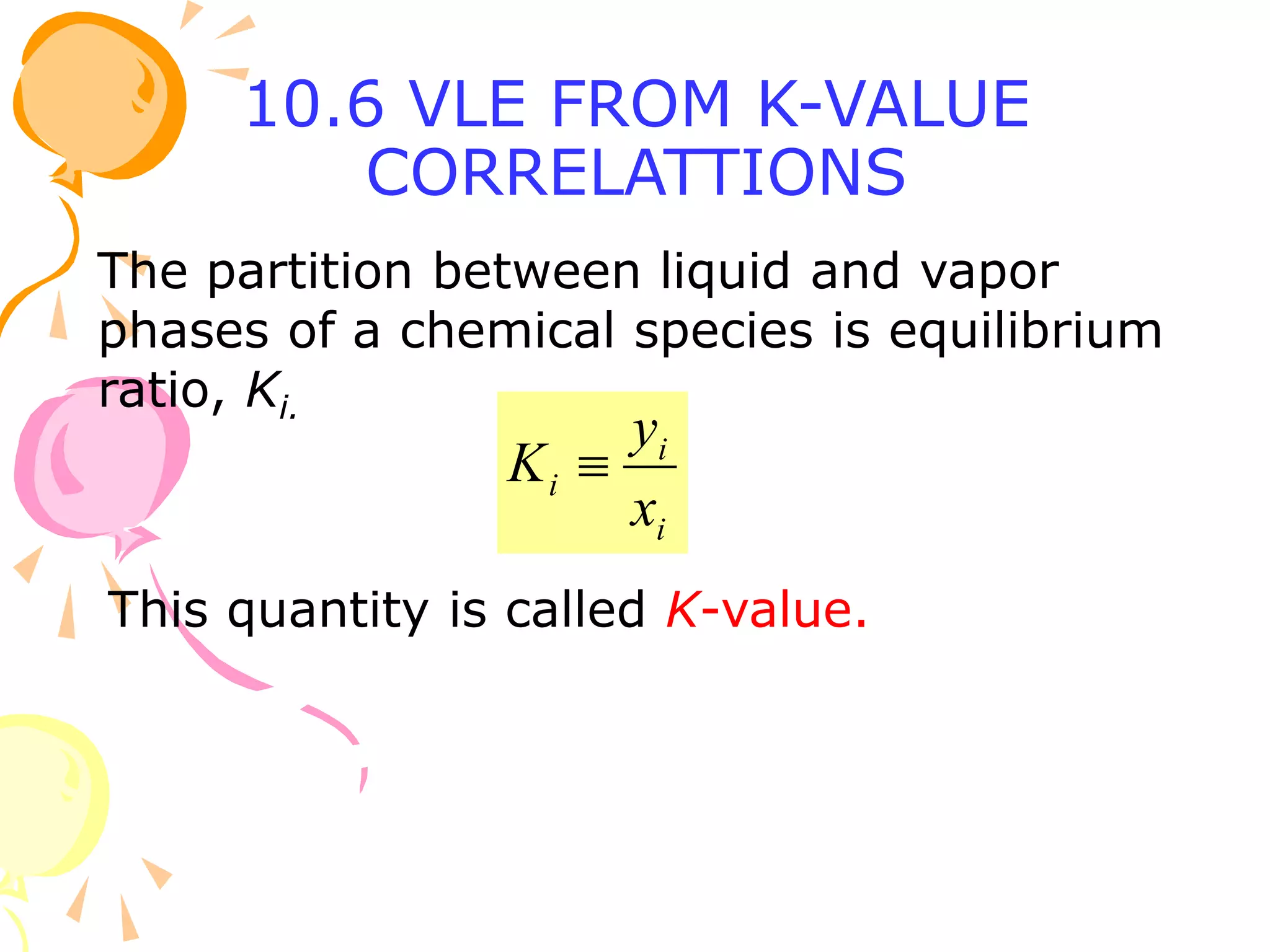
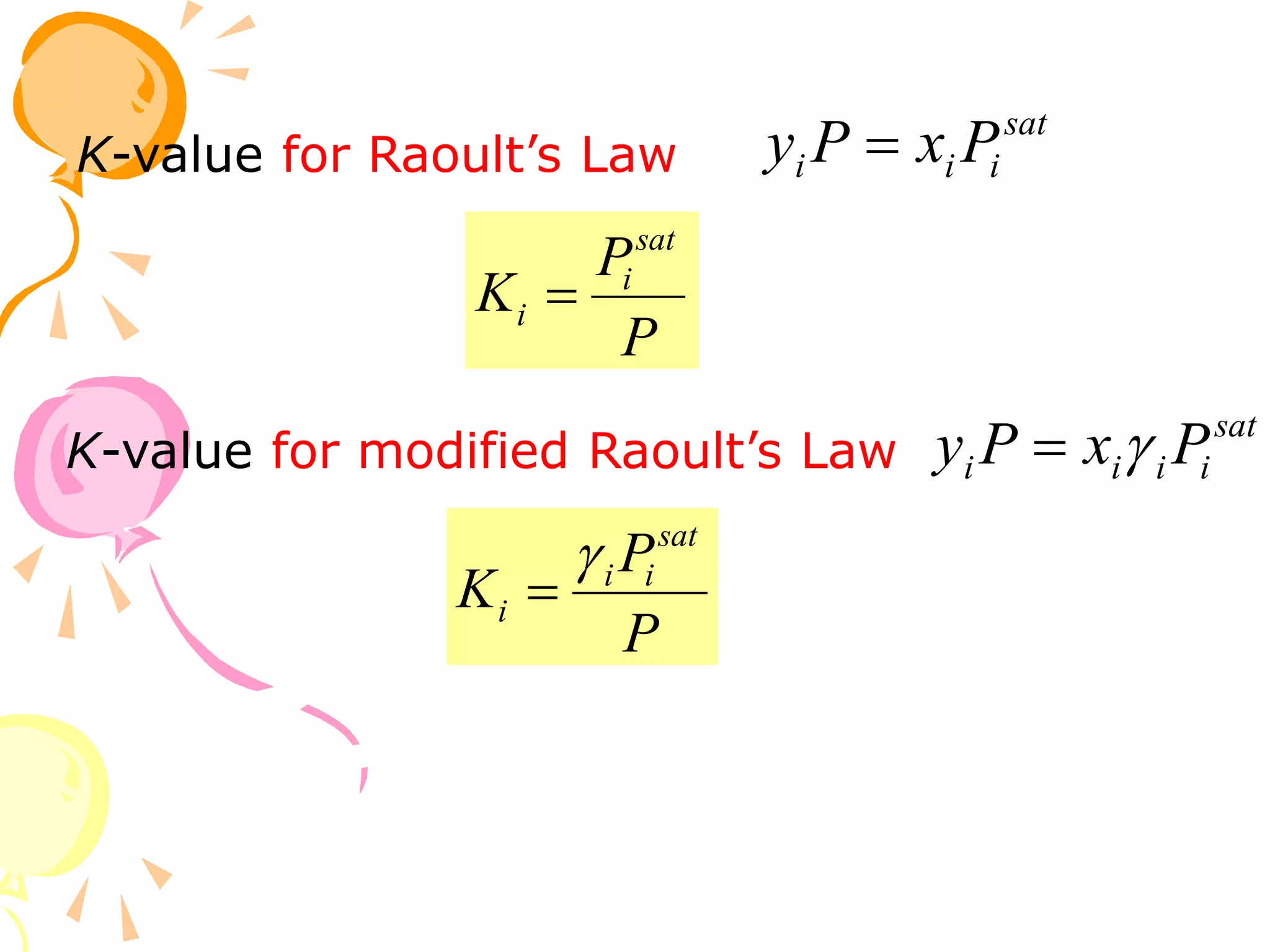
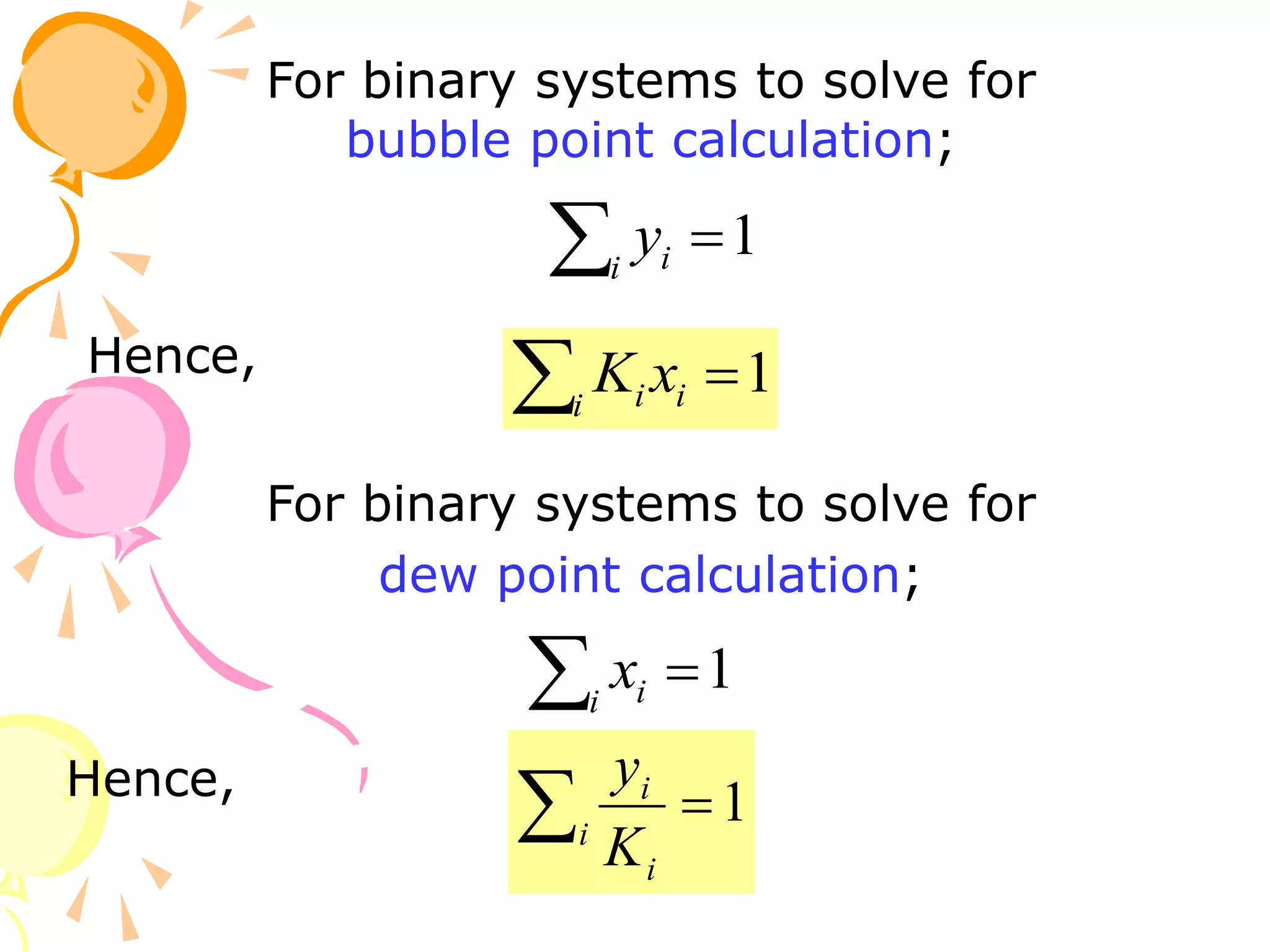
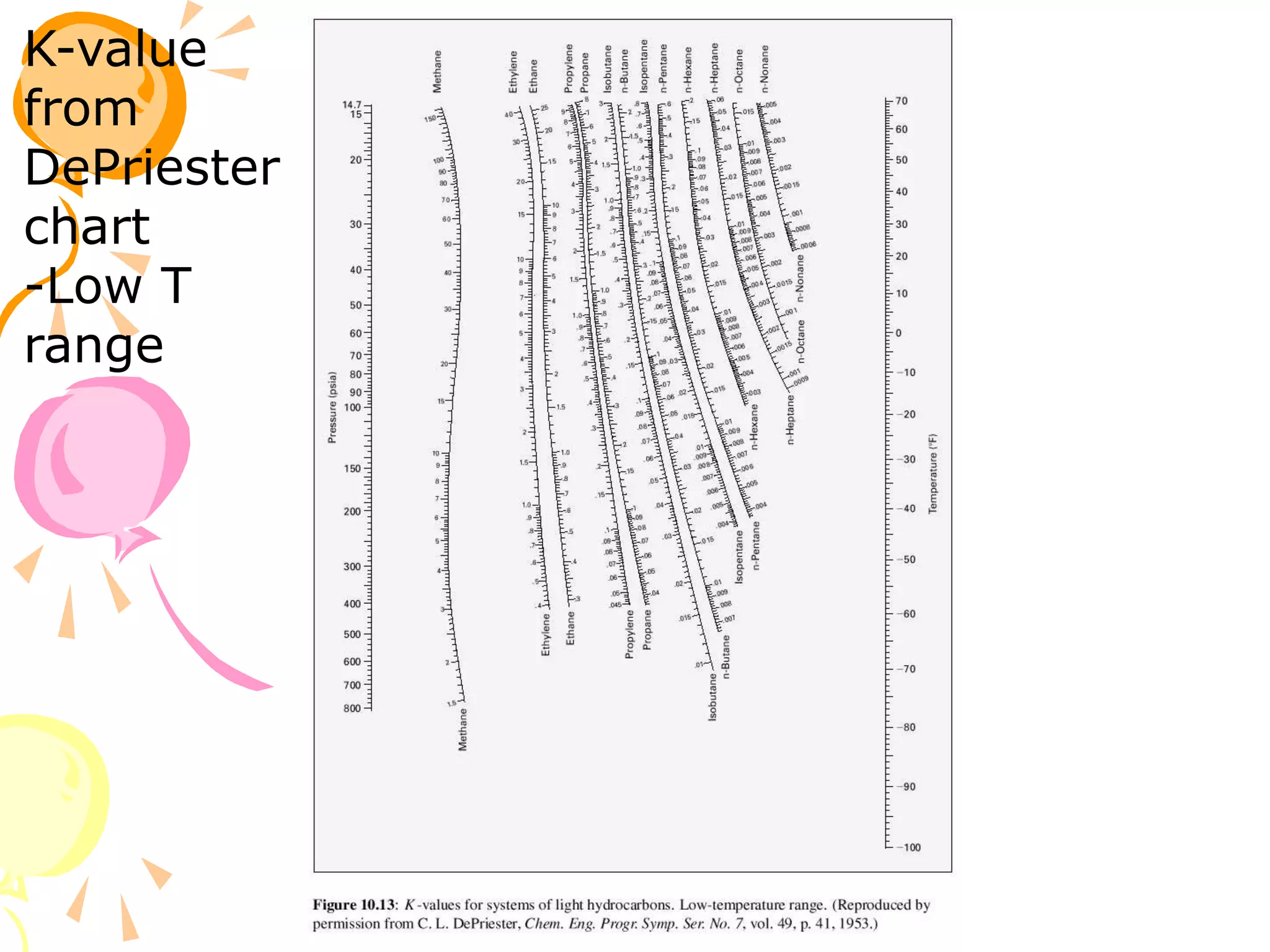
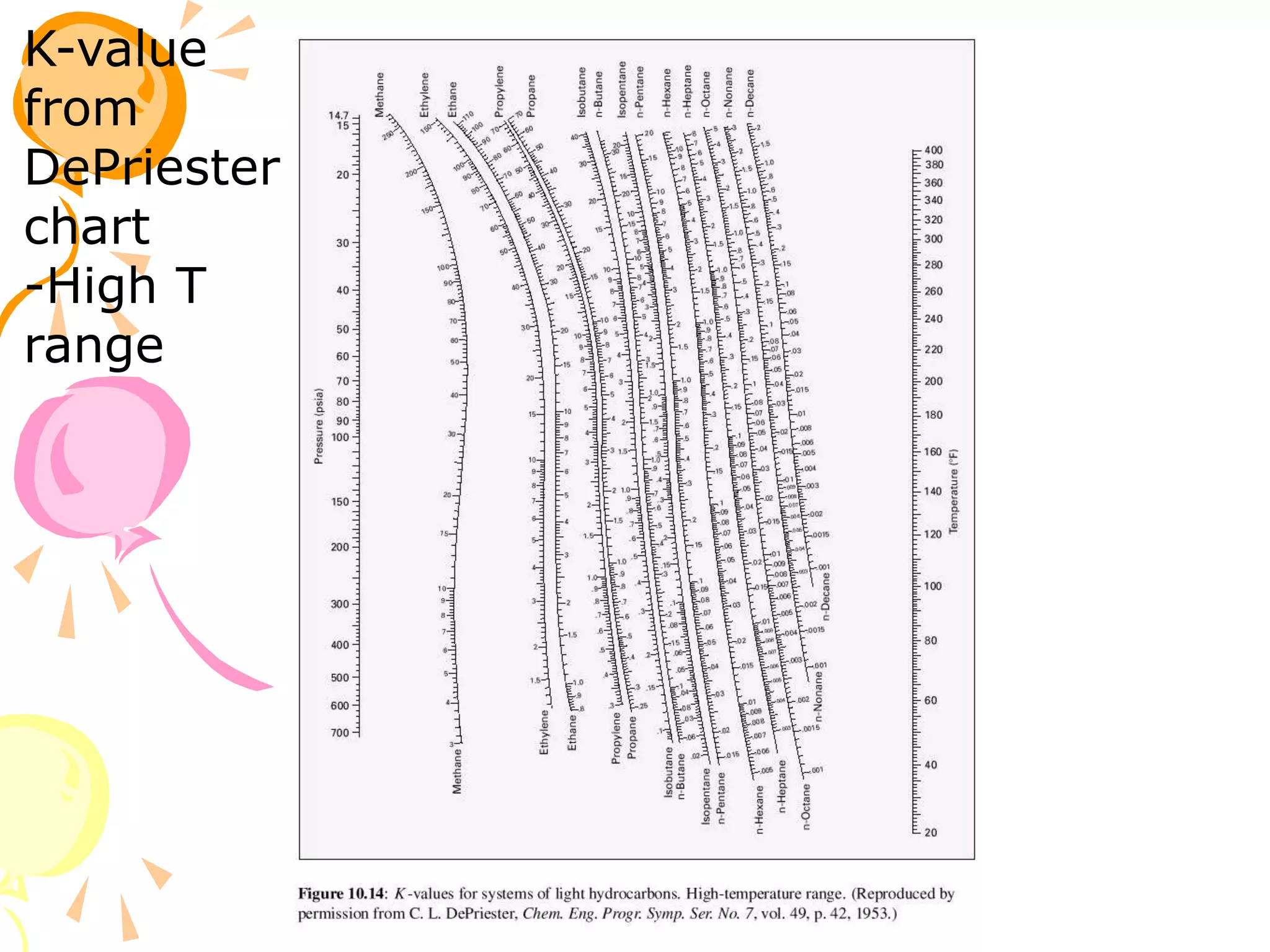
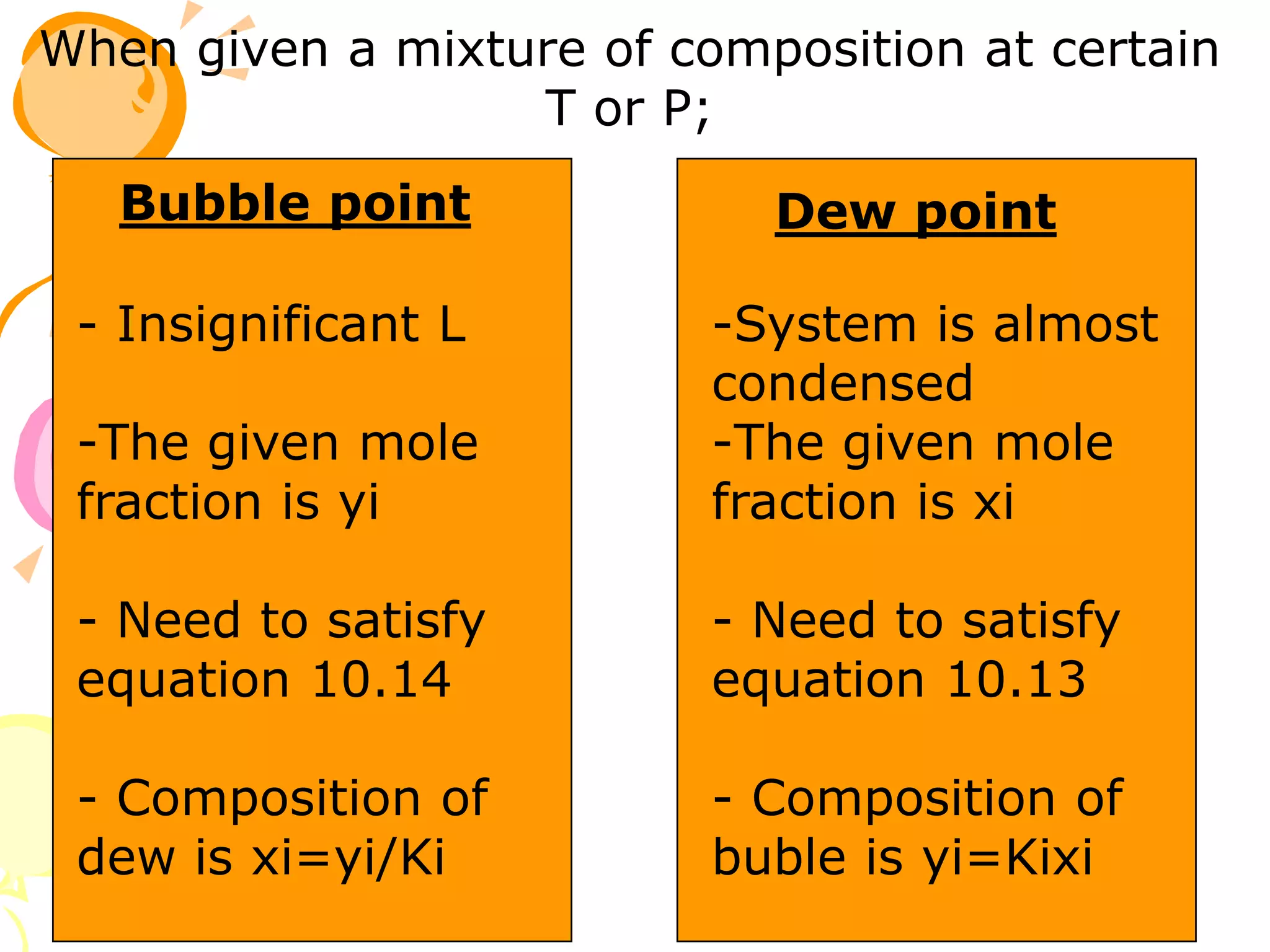
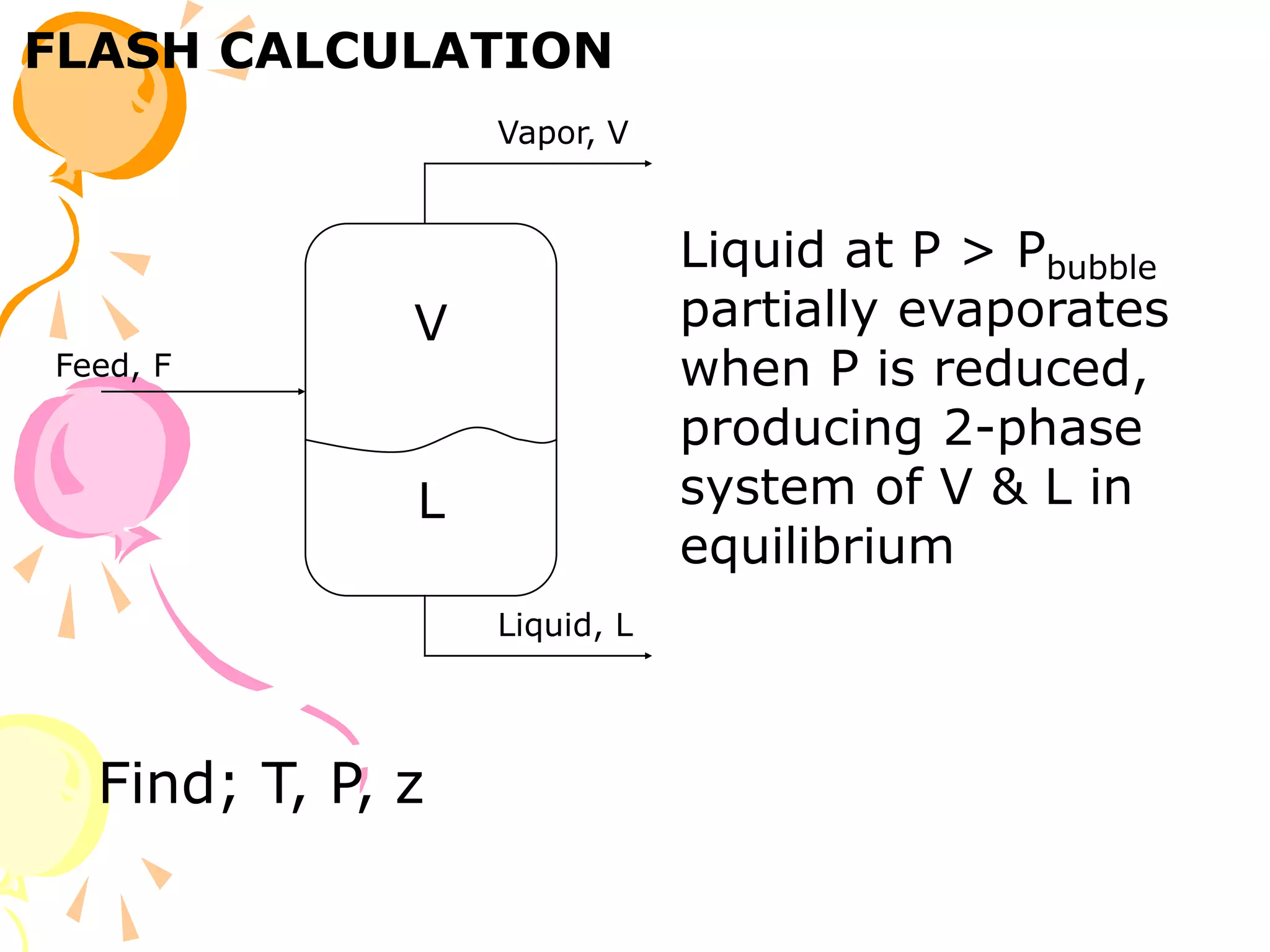
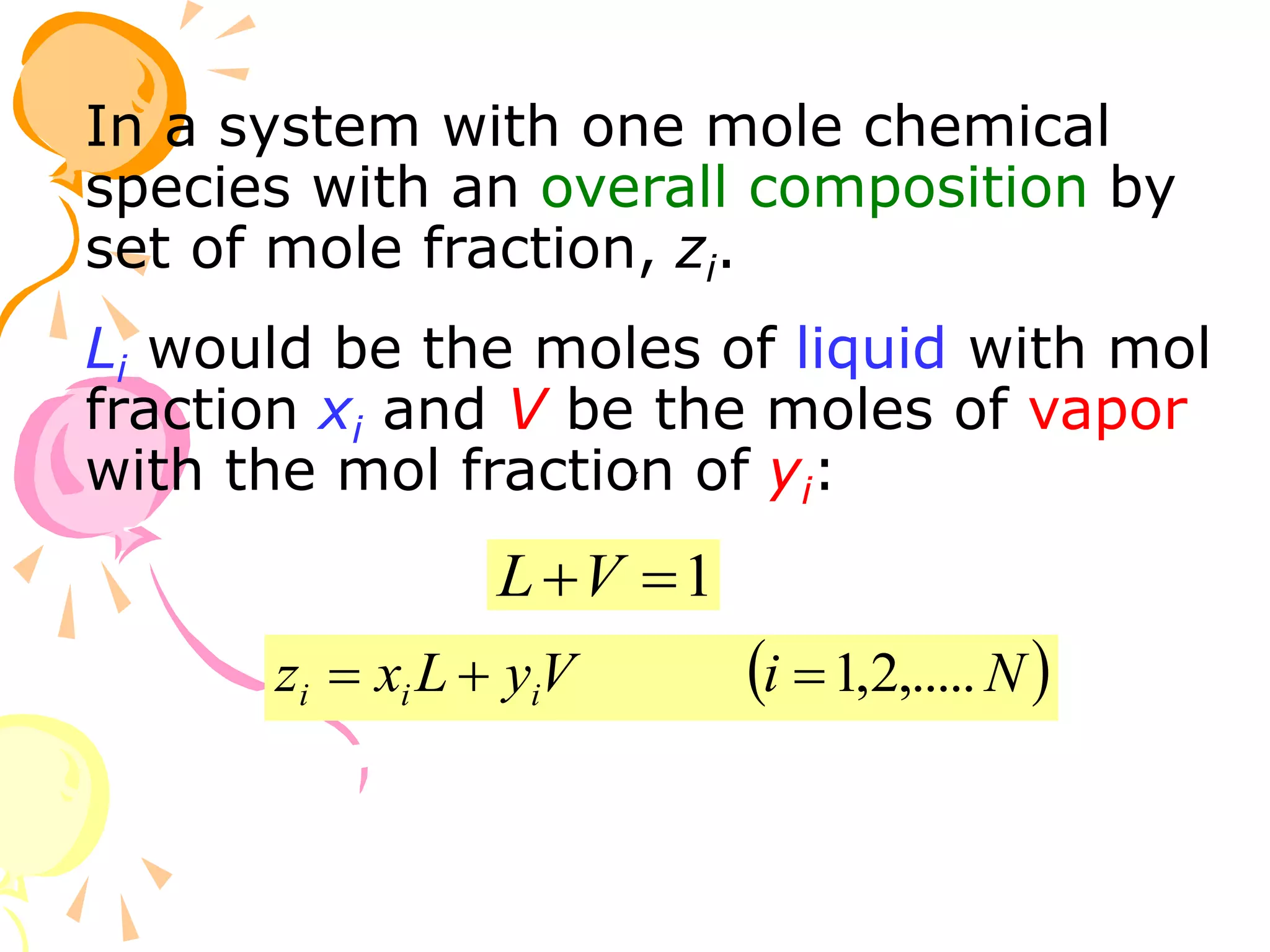
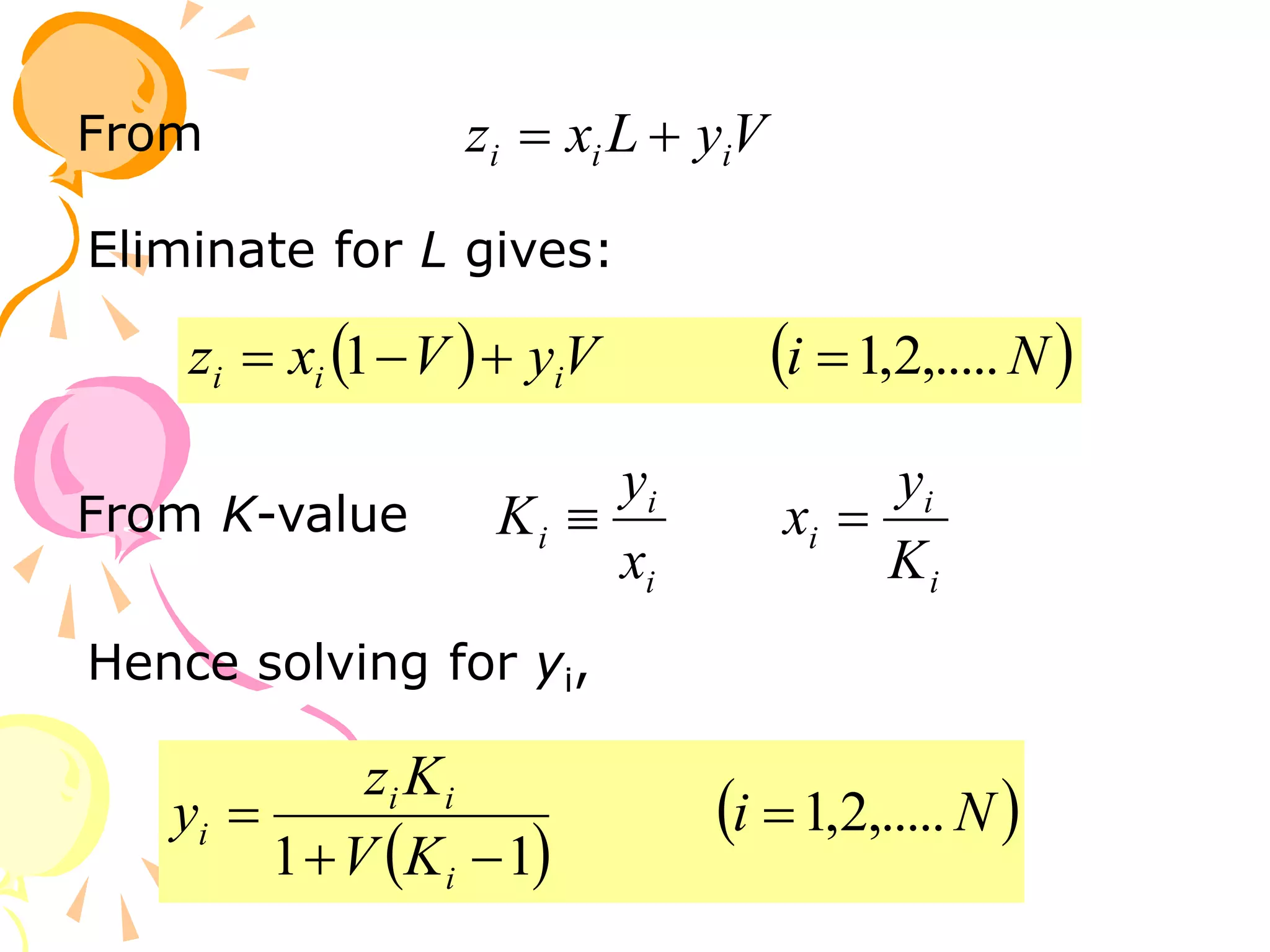
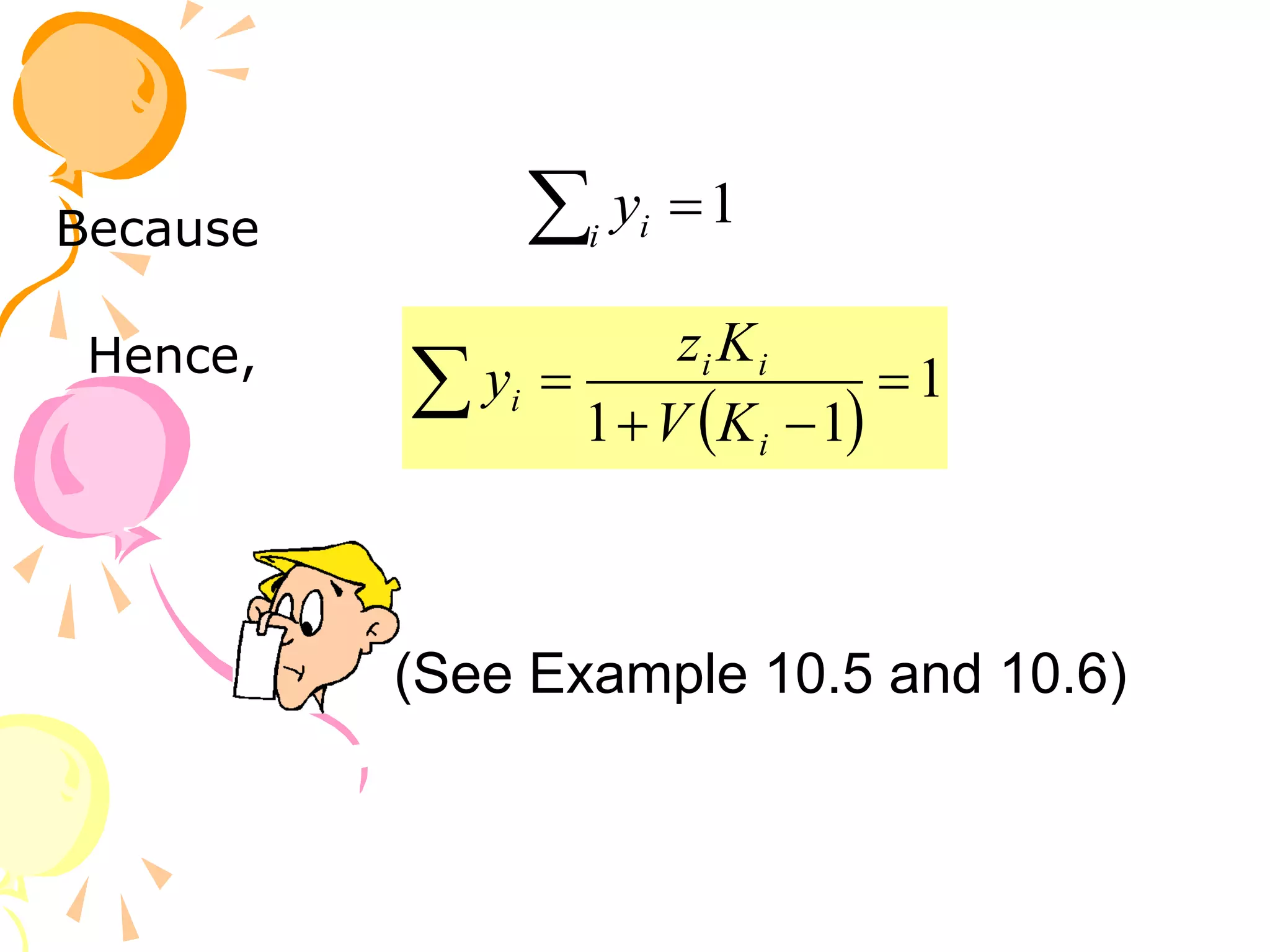
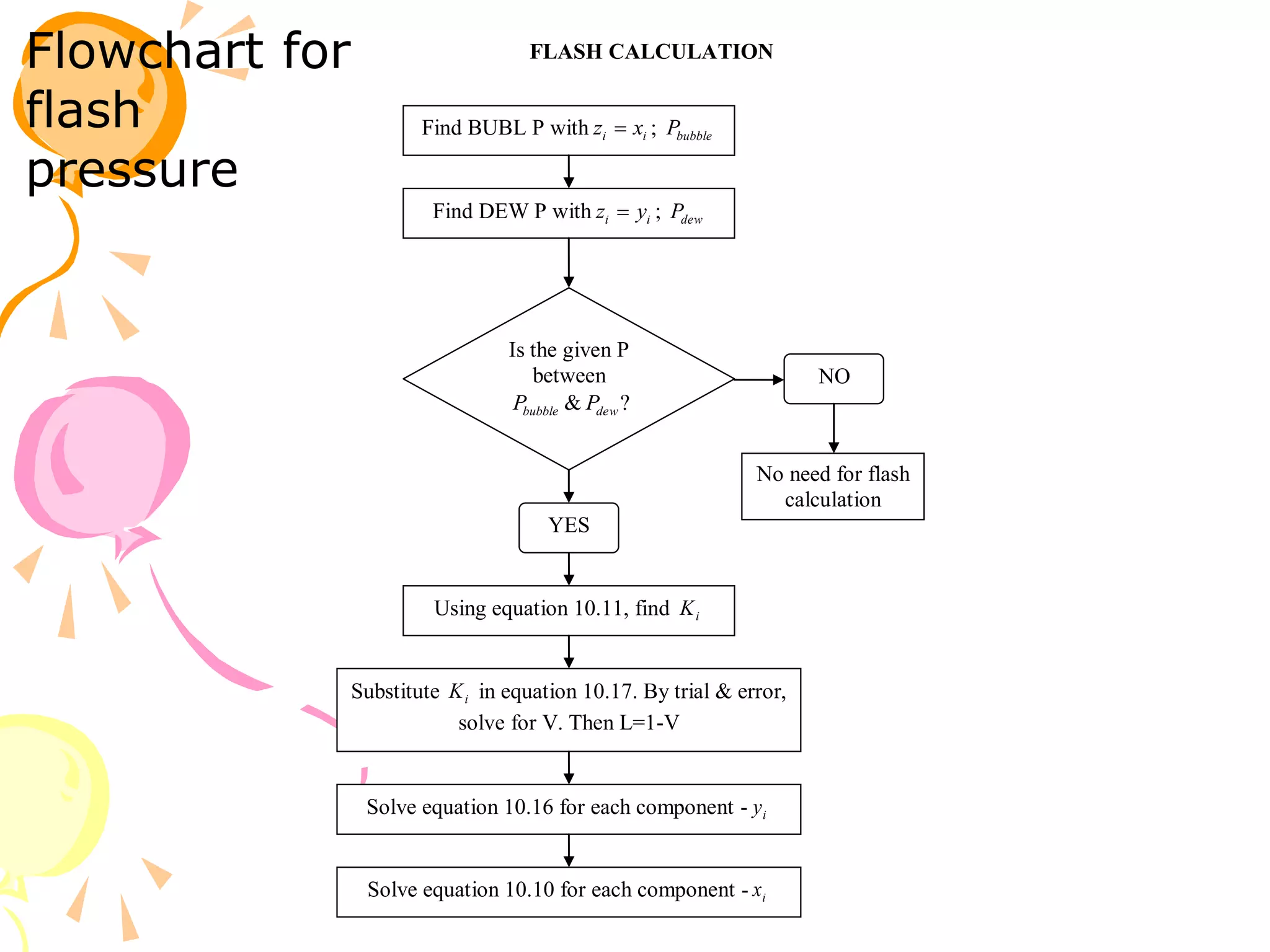
3. Examples are provided to demonstrate calculating bubble point, dew point, and equilibrium conditions using these models. Modified Raoult's law is also introduced, which accounts for non-ideality in the liquid phase using activity coefficients.