The document describes the second law of thermodynamics and reversible processes involving perfect gases on temperature-entropy (T-s) diagrams. It discusses:
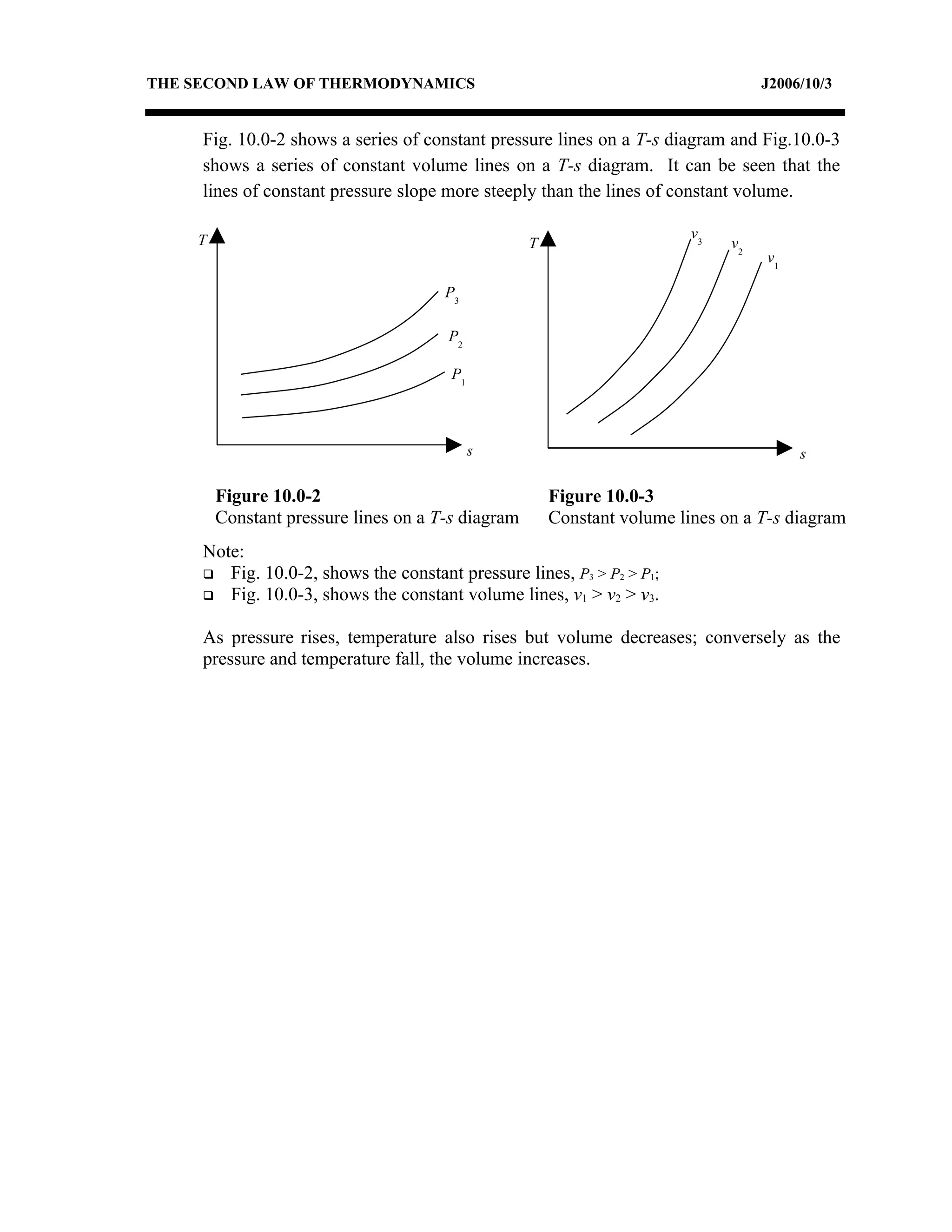
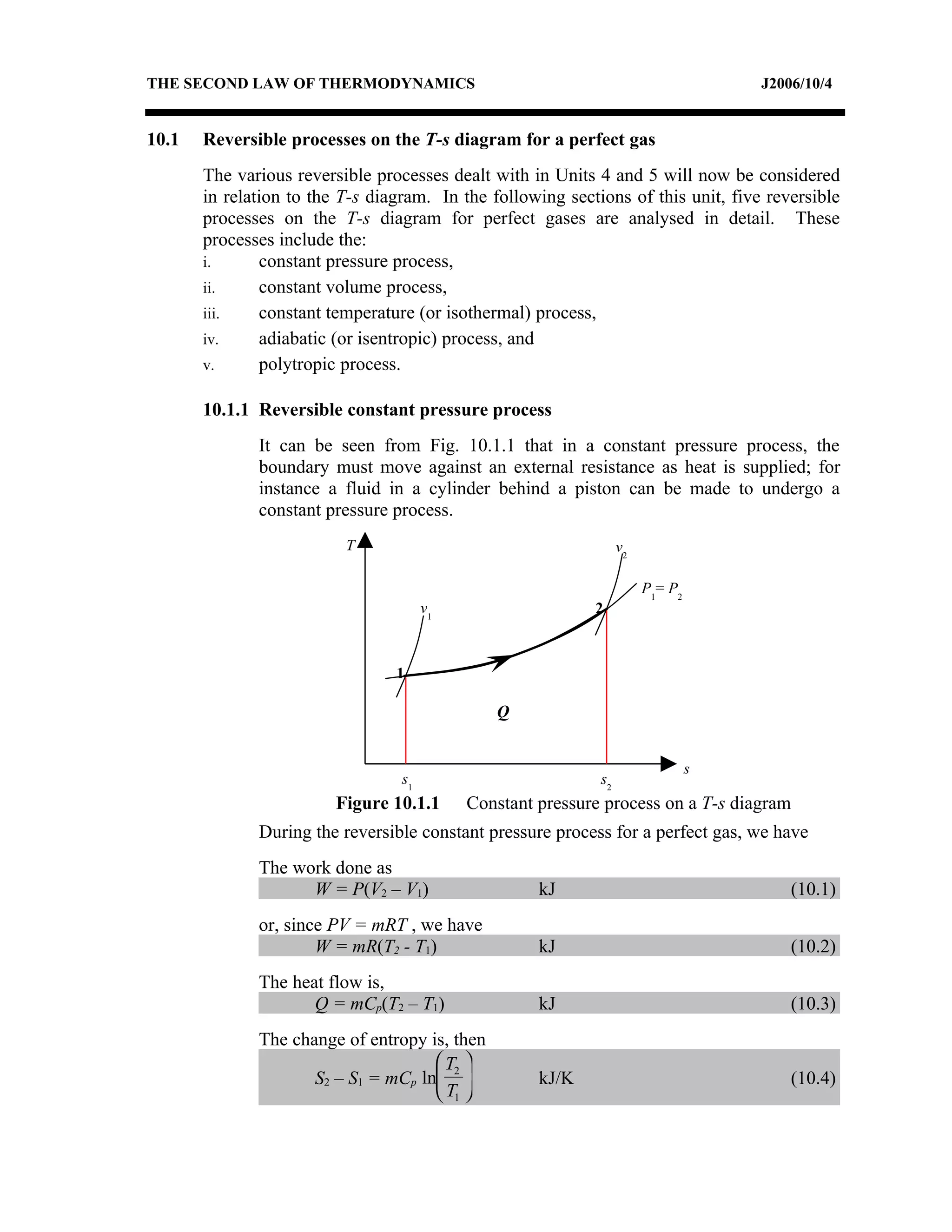
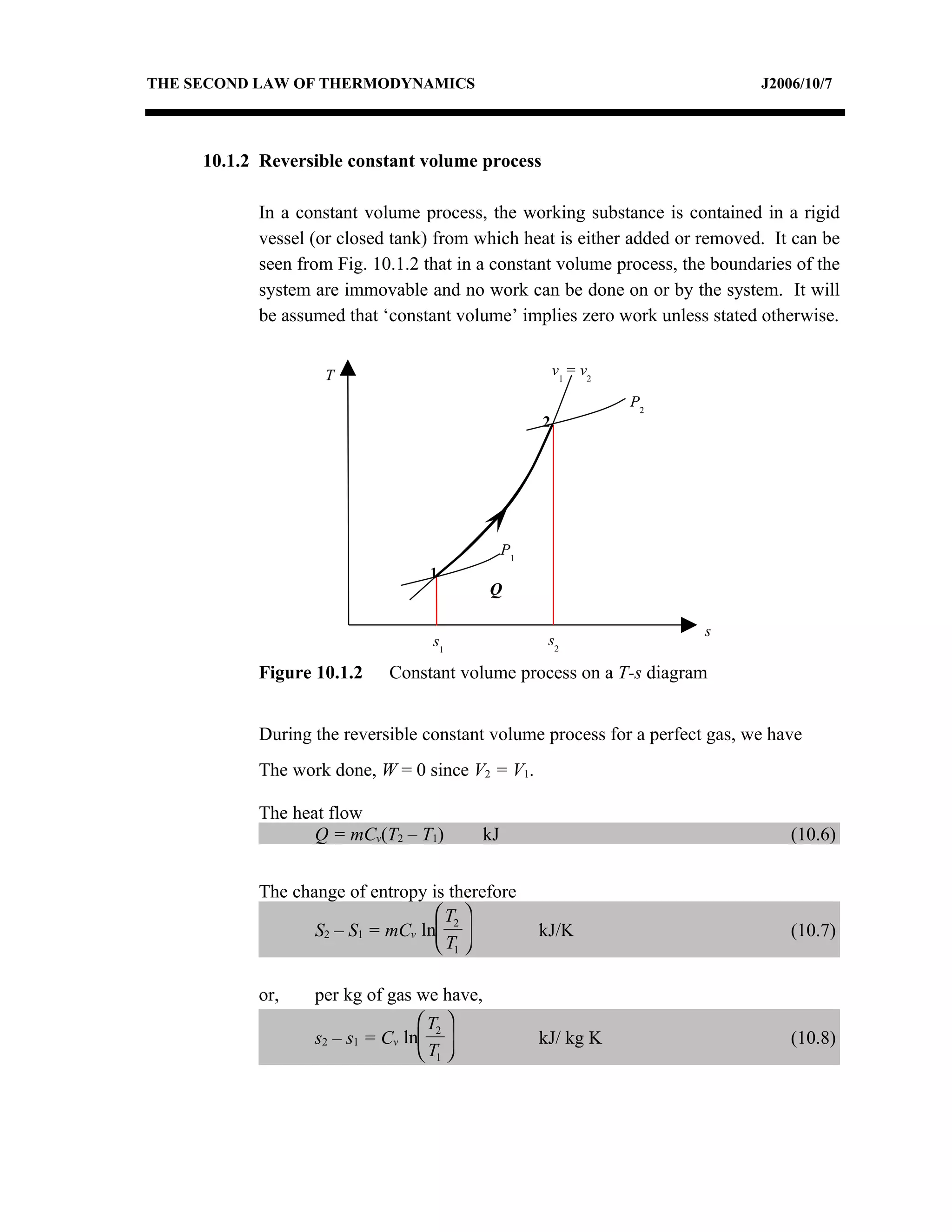
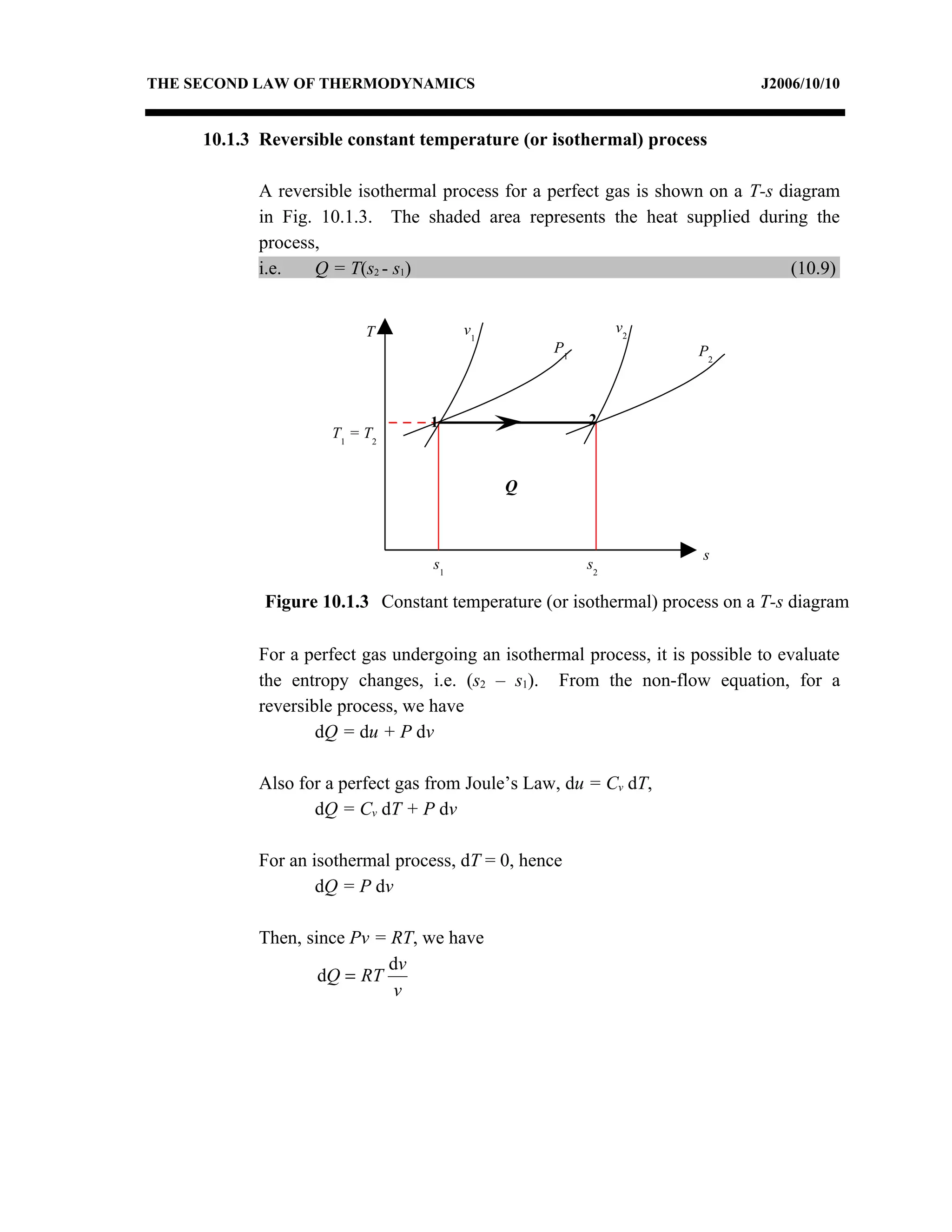
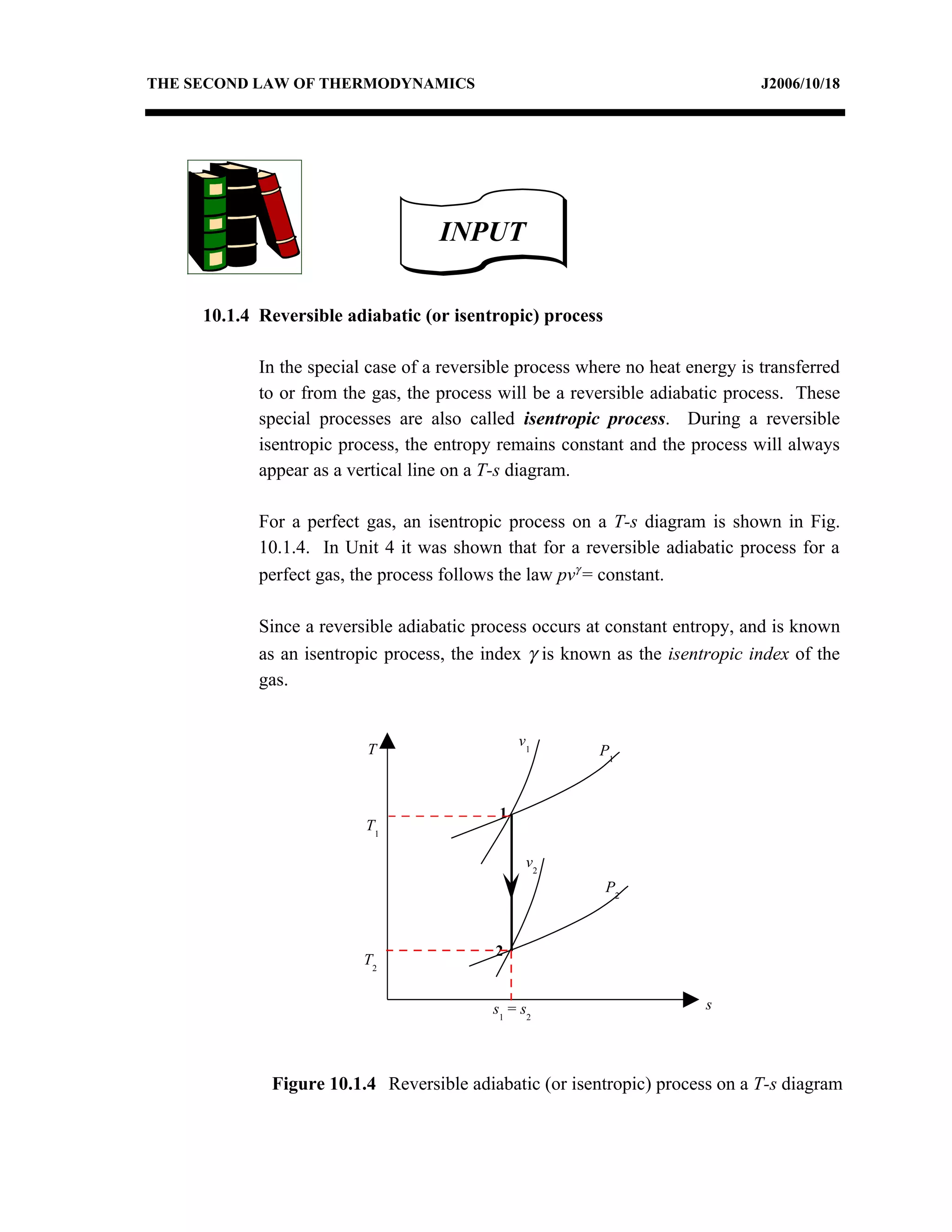
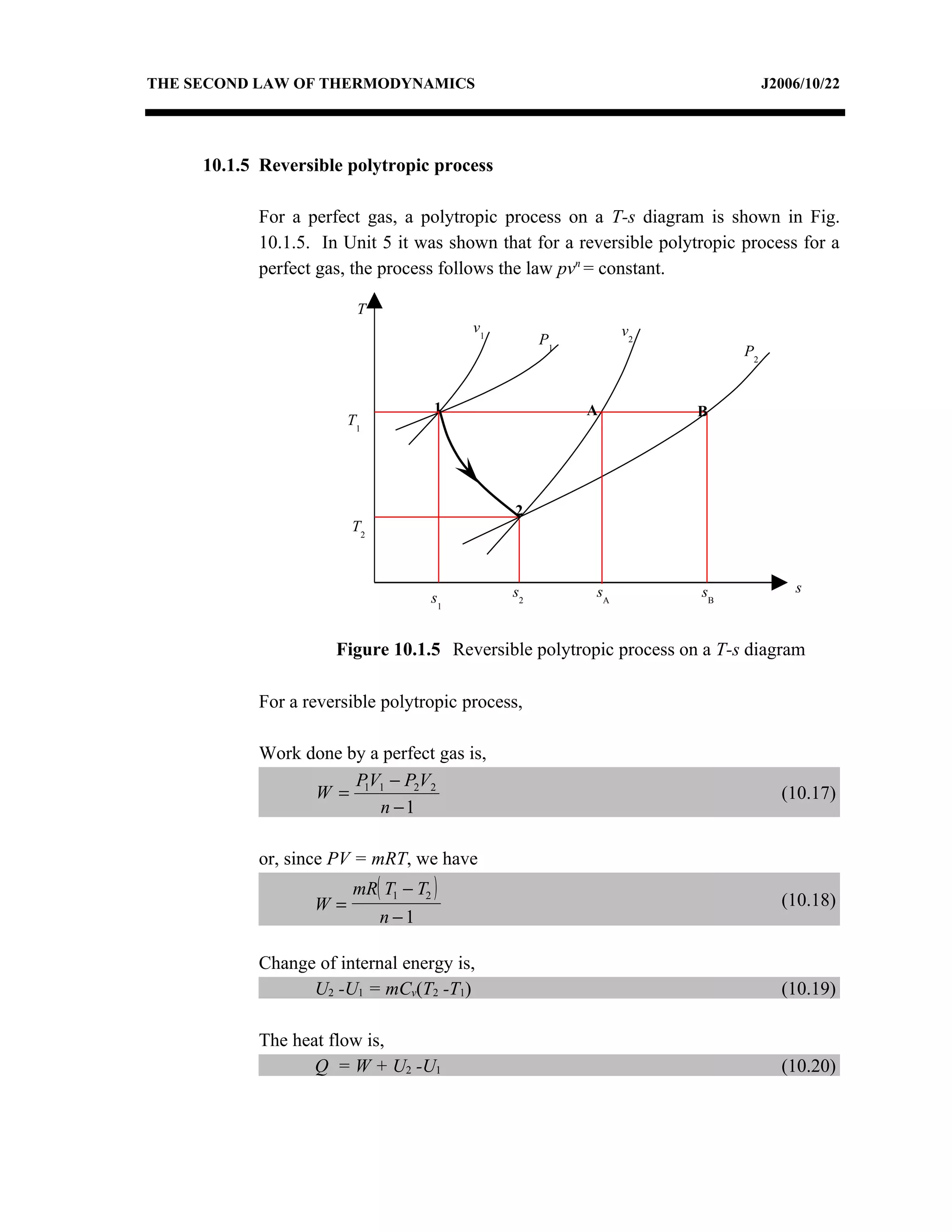
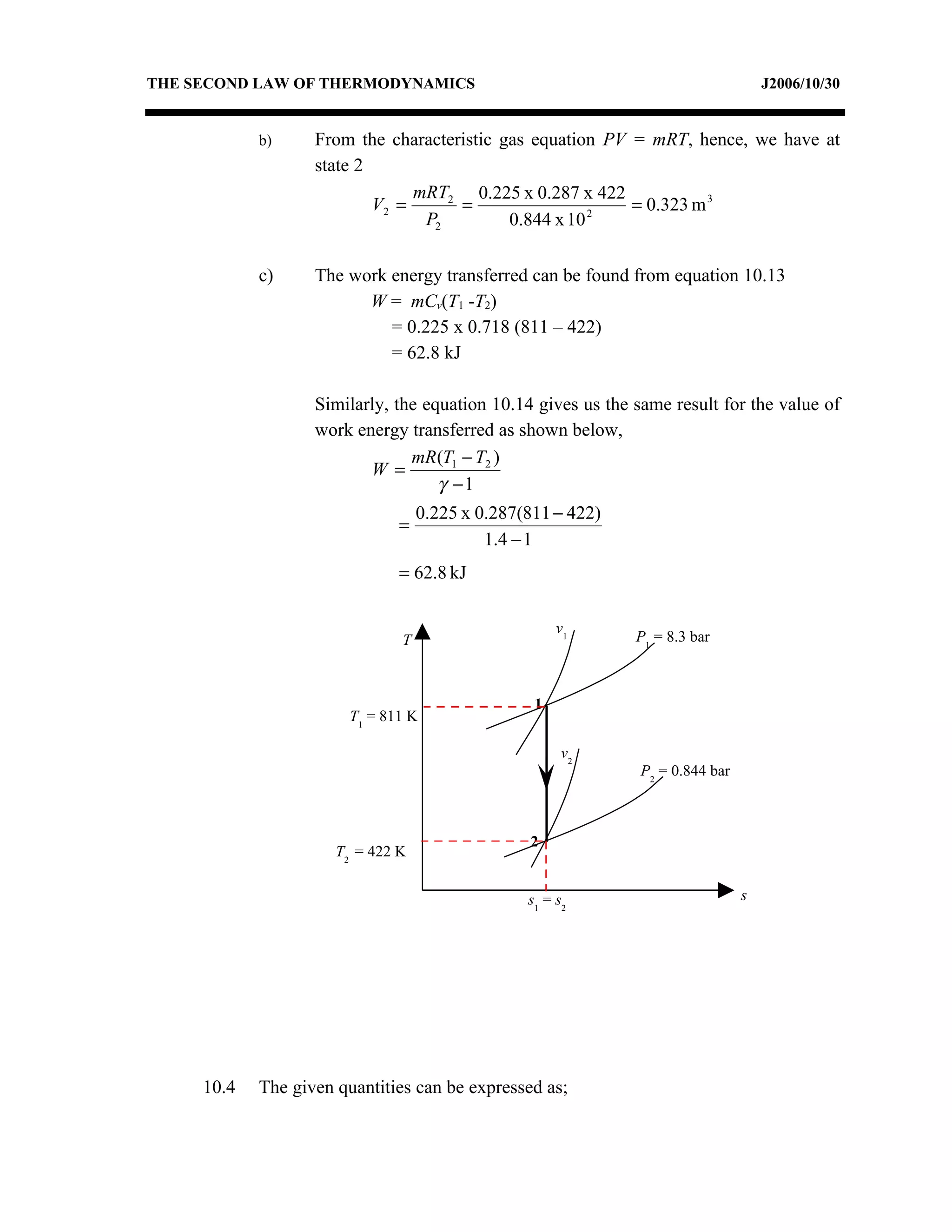
1) Constant pressure, volume, temperature, and adiabatic processes on T-s diagrams, with constant pressure lines sloping more steeply than constant volume lines.
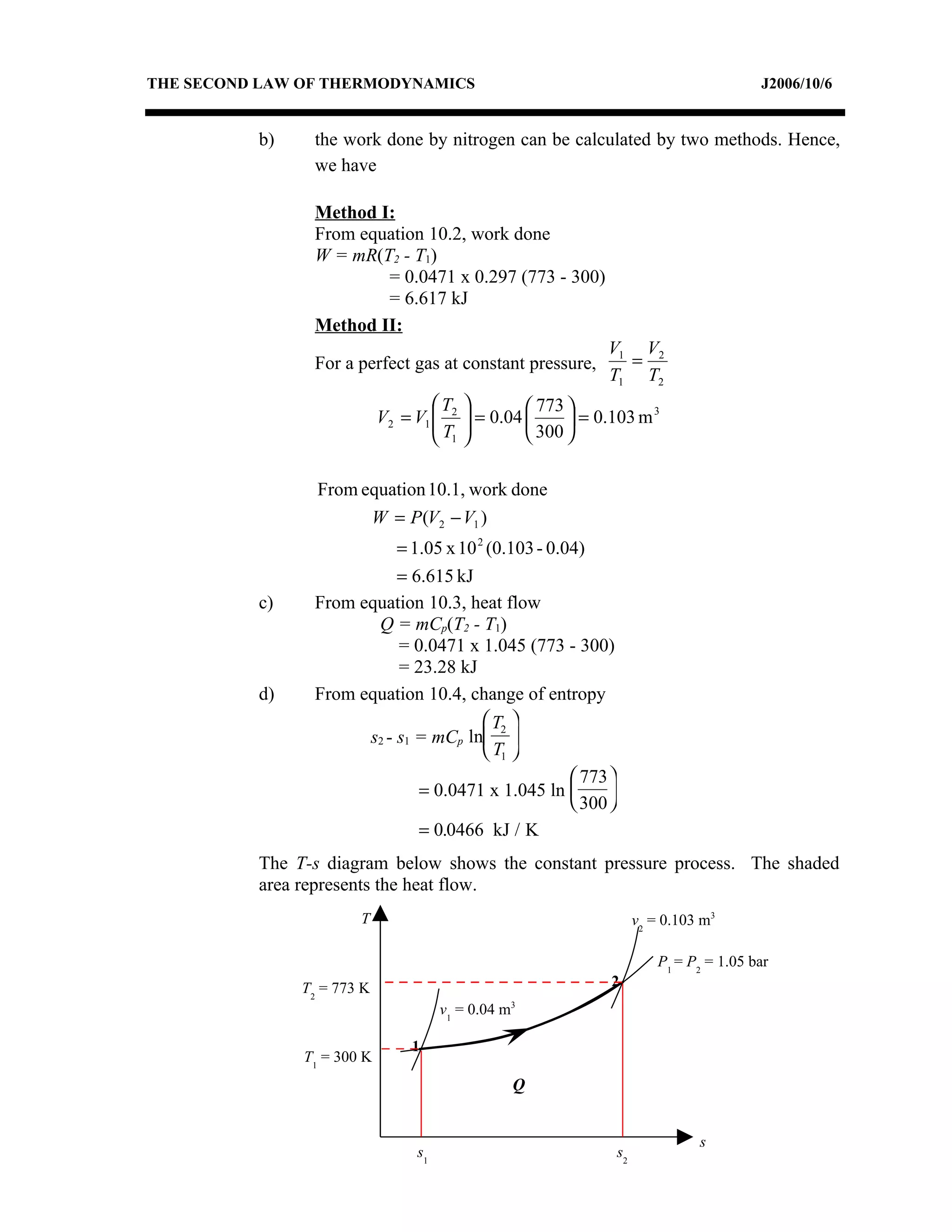
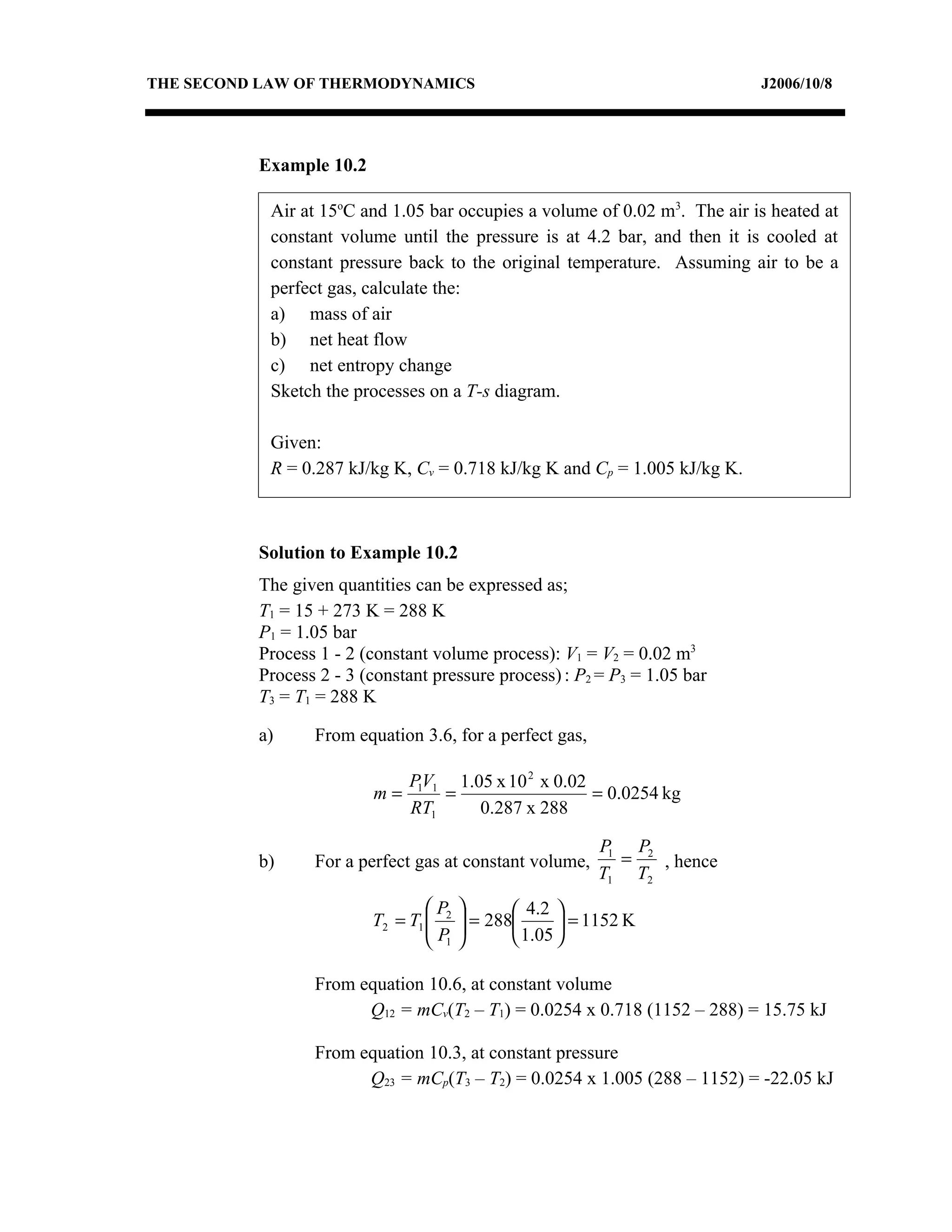
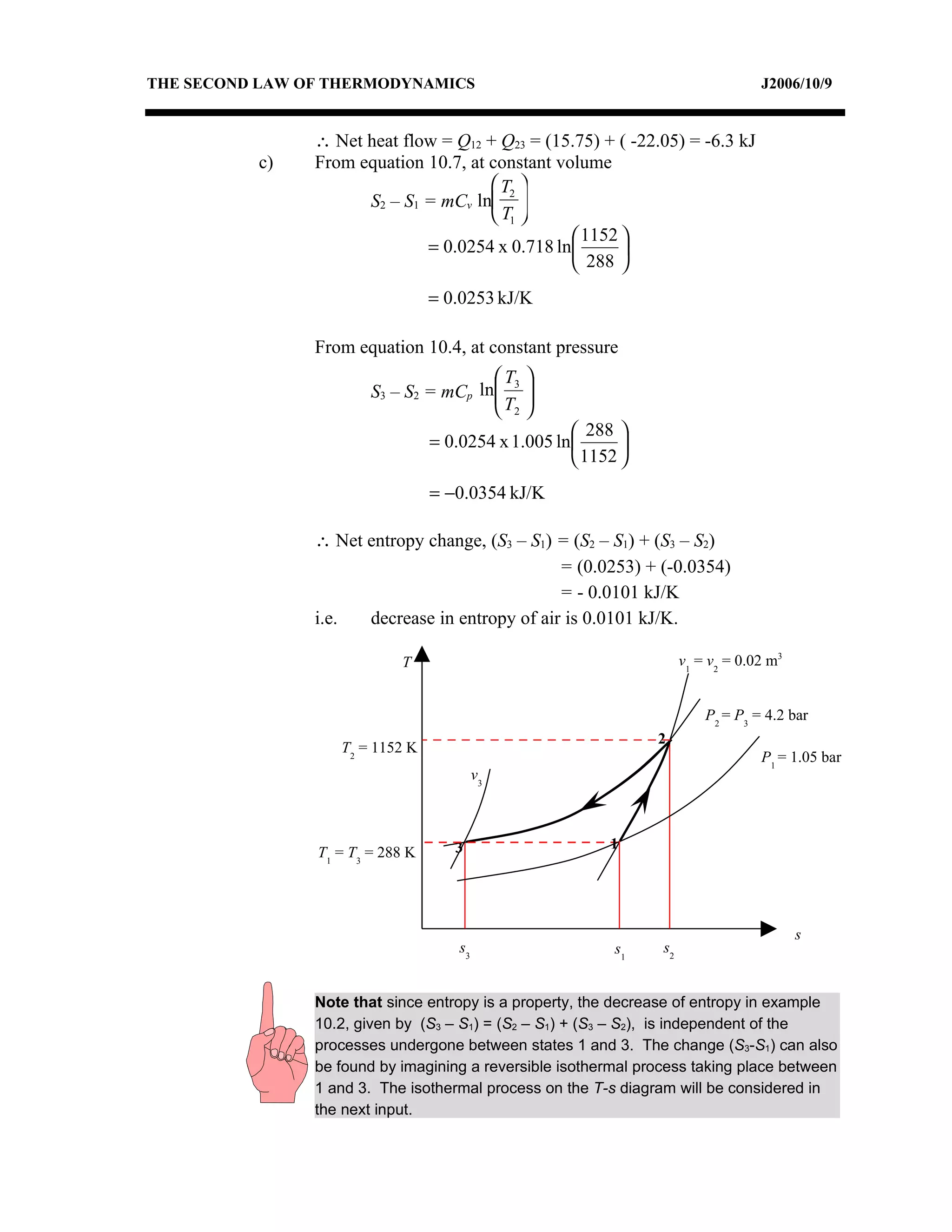
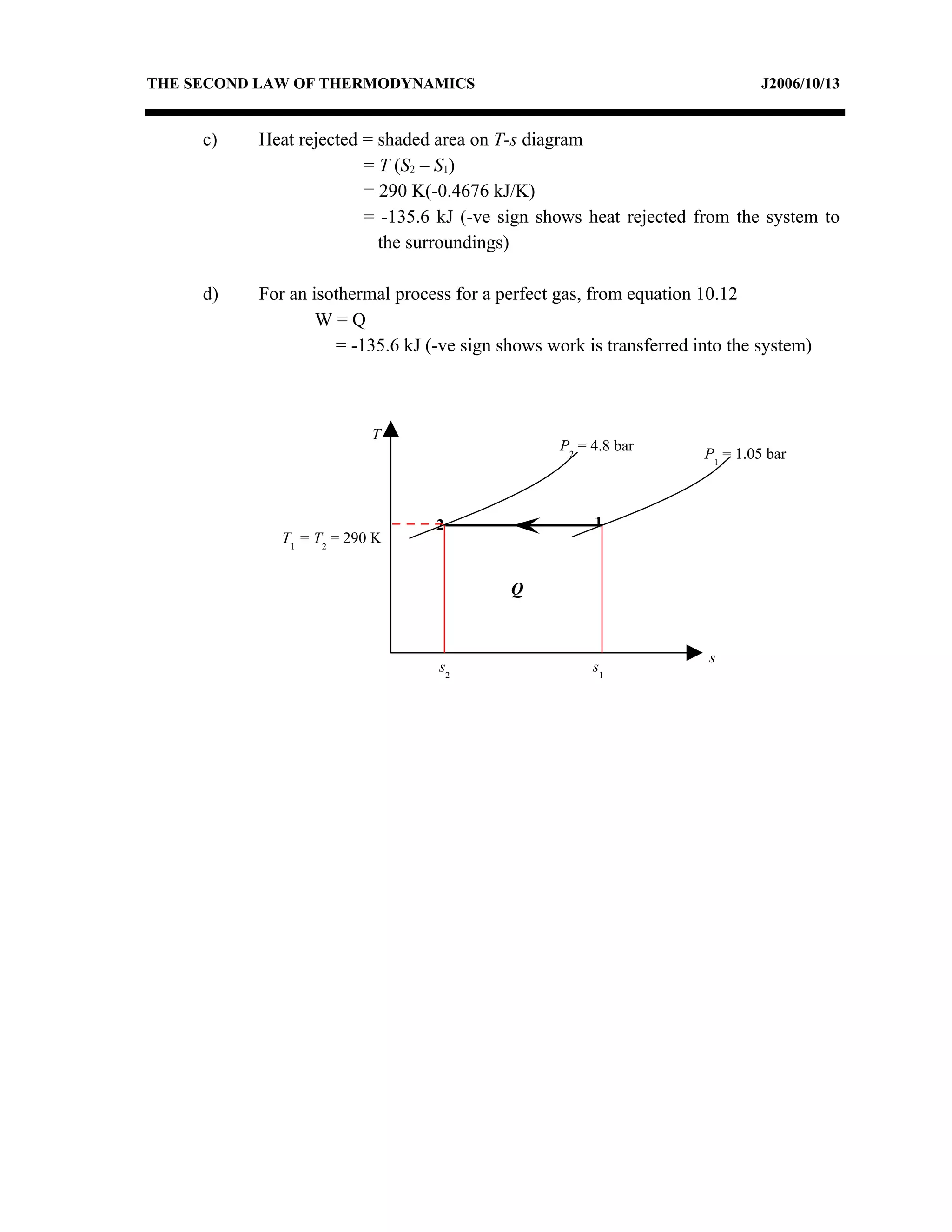
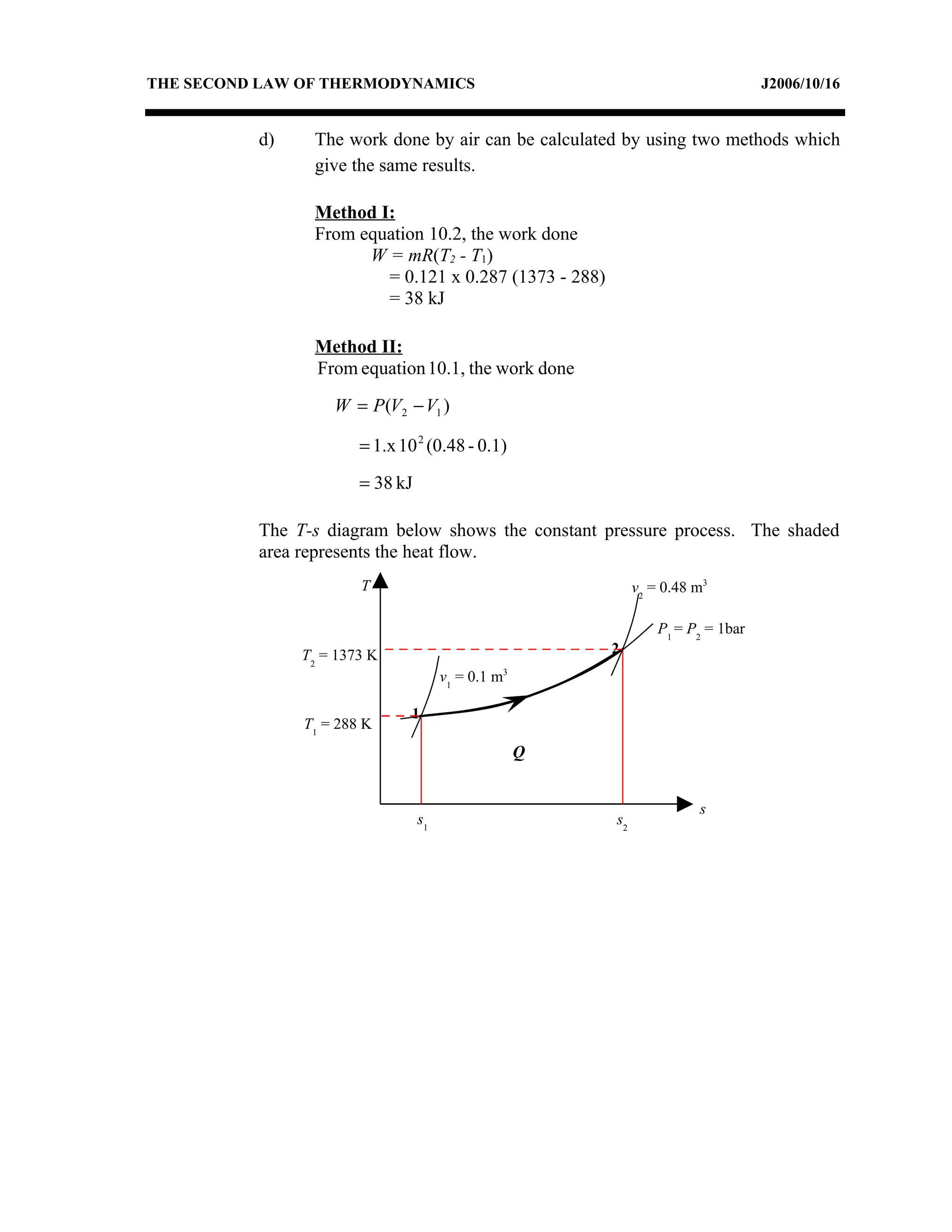
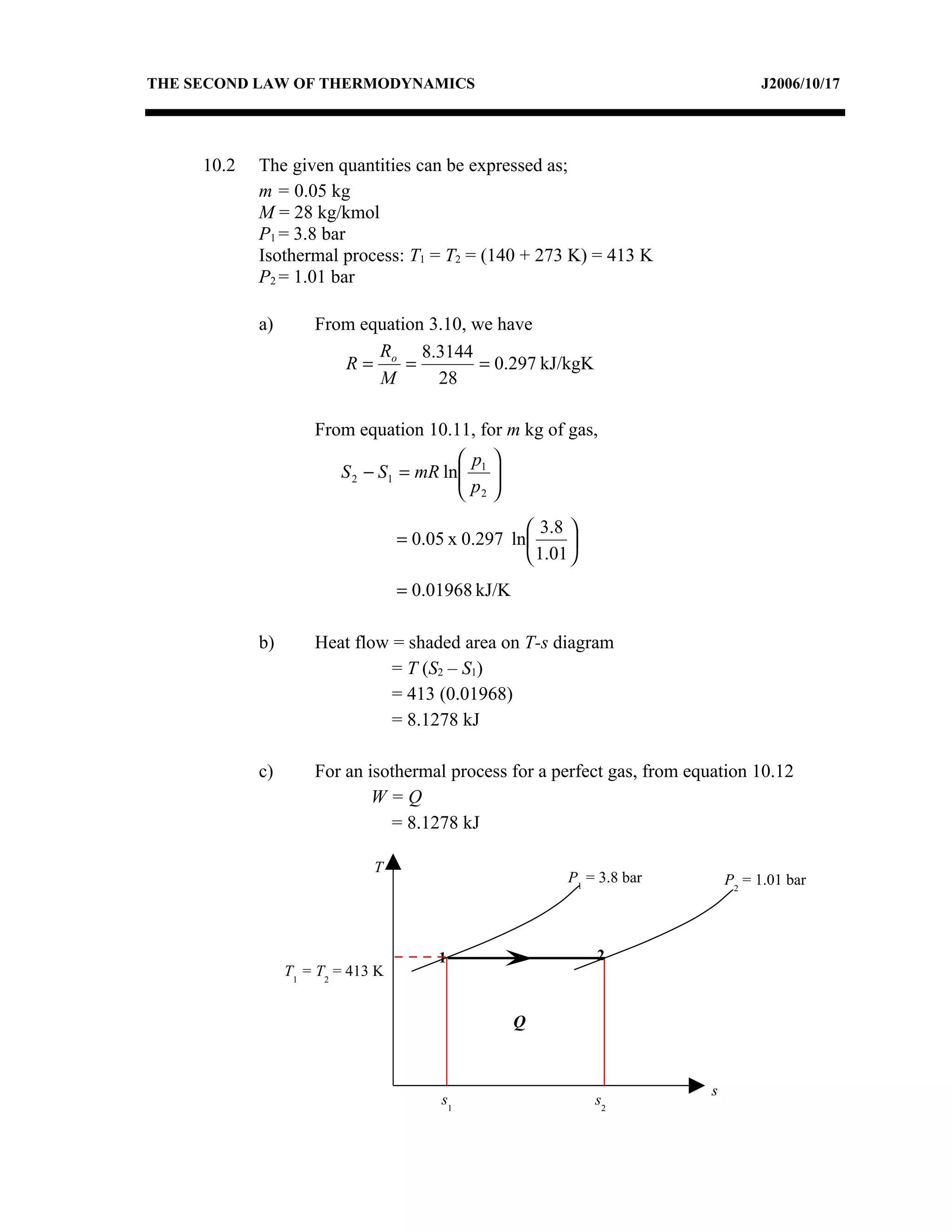
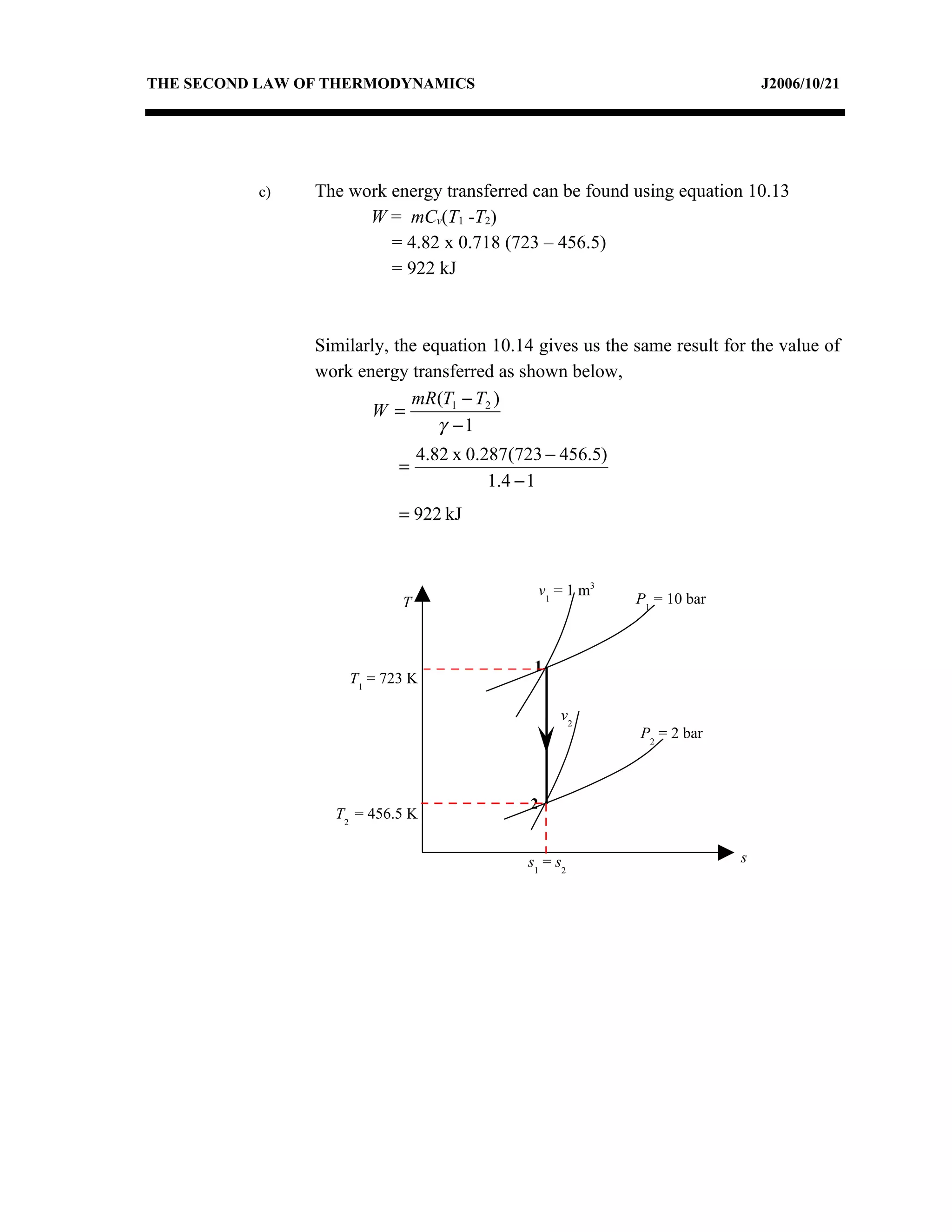
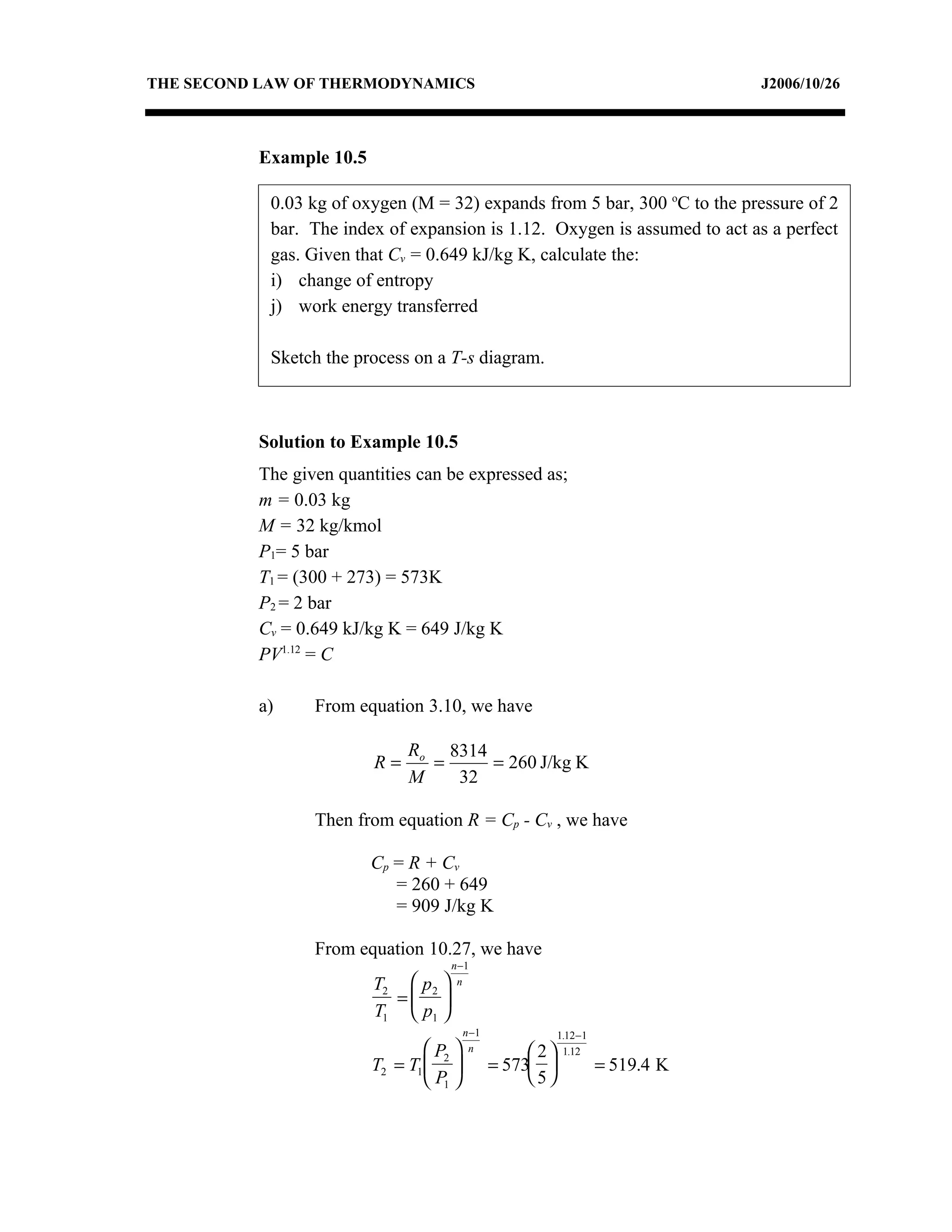
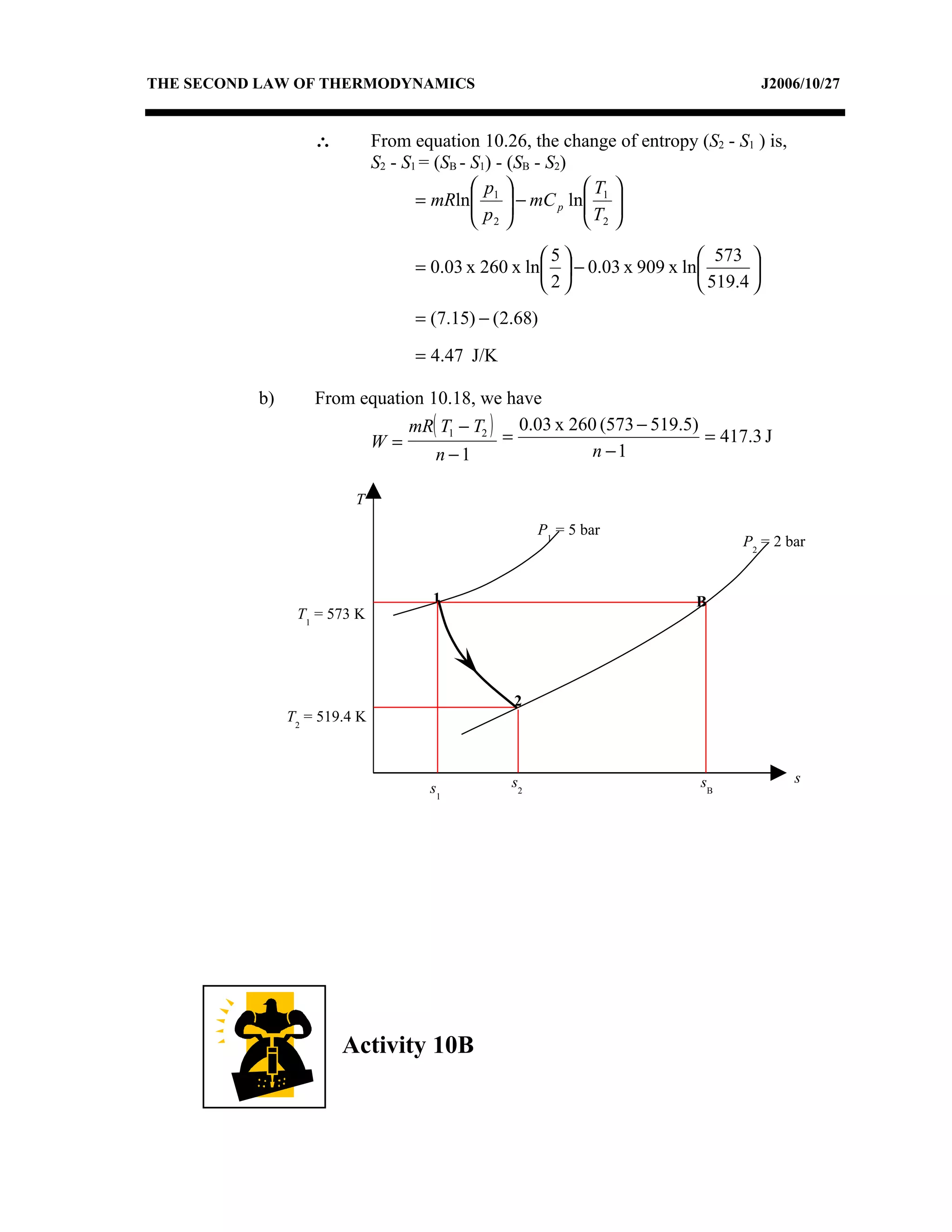
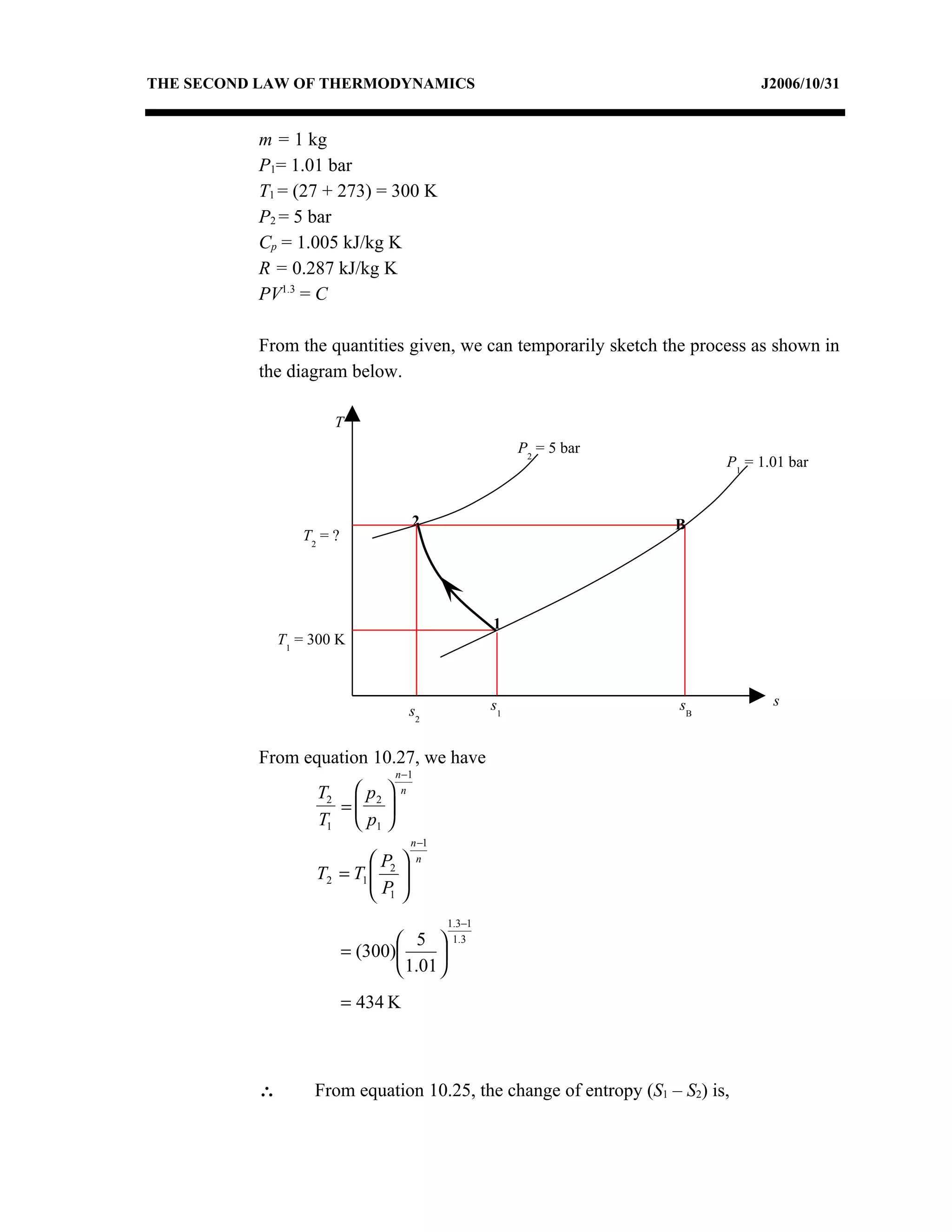
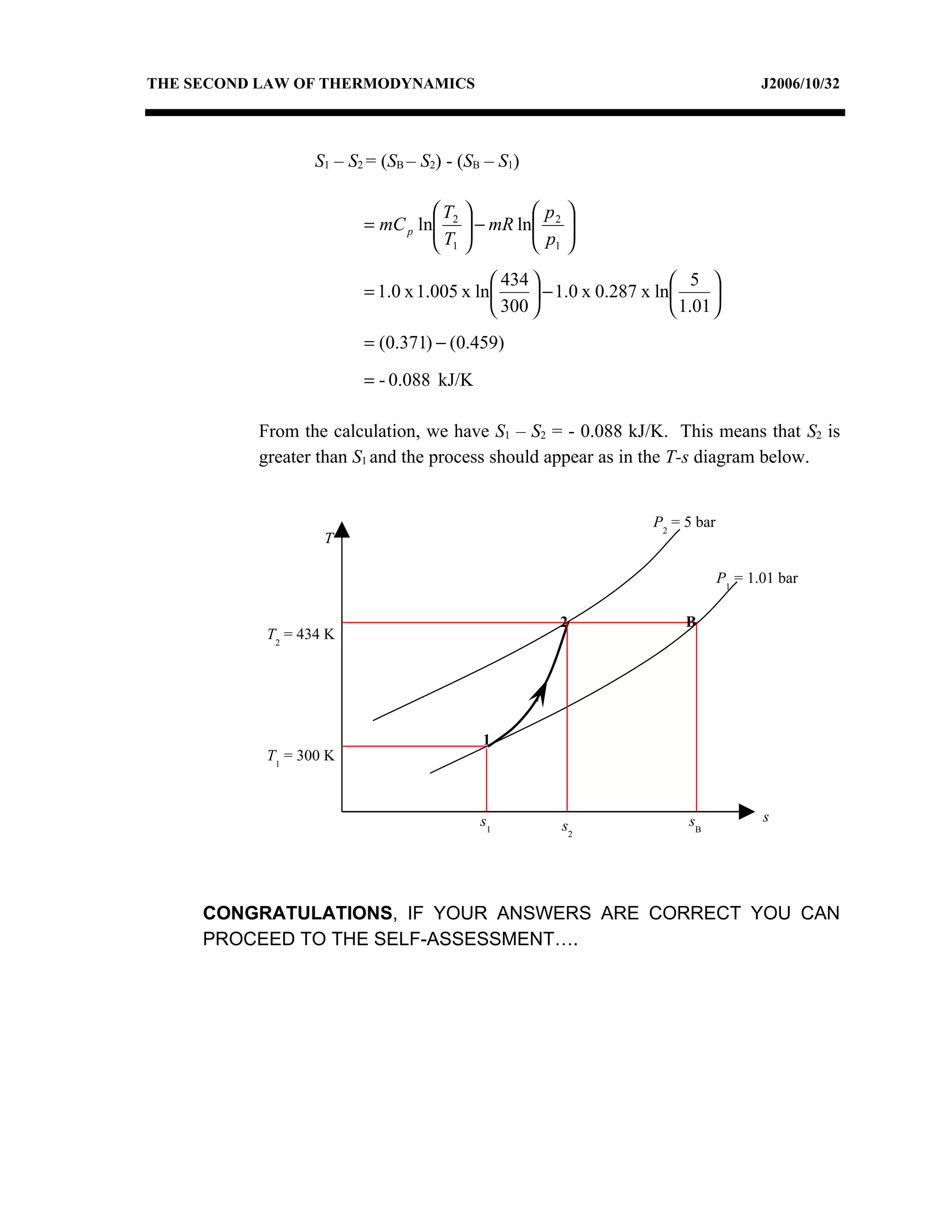
2) Analyzing a example problem involving a constant pressure expansion of nitrogen gas, calculating work, heat, entropy change, and sketching the process on a T-s diagram.
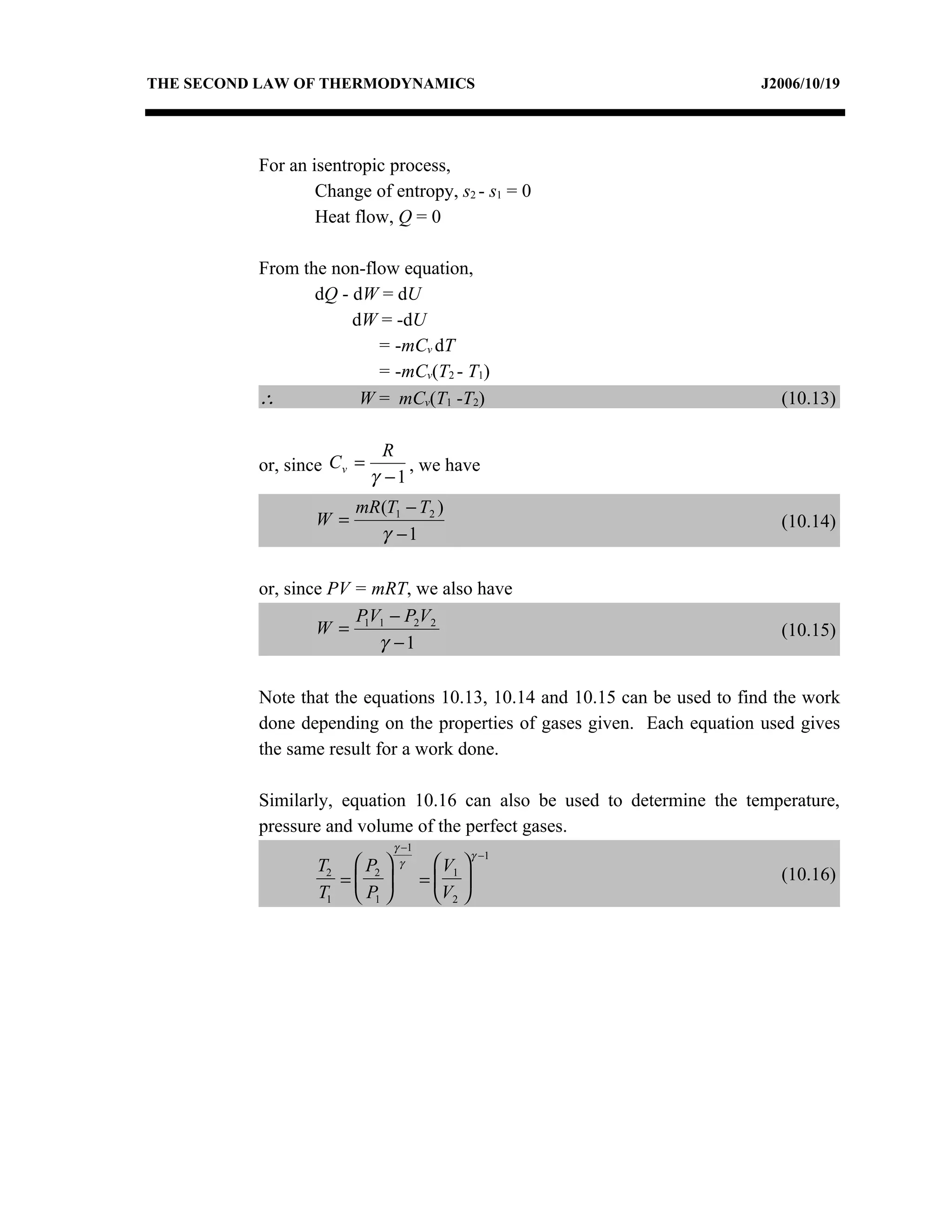
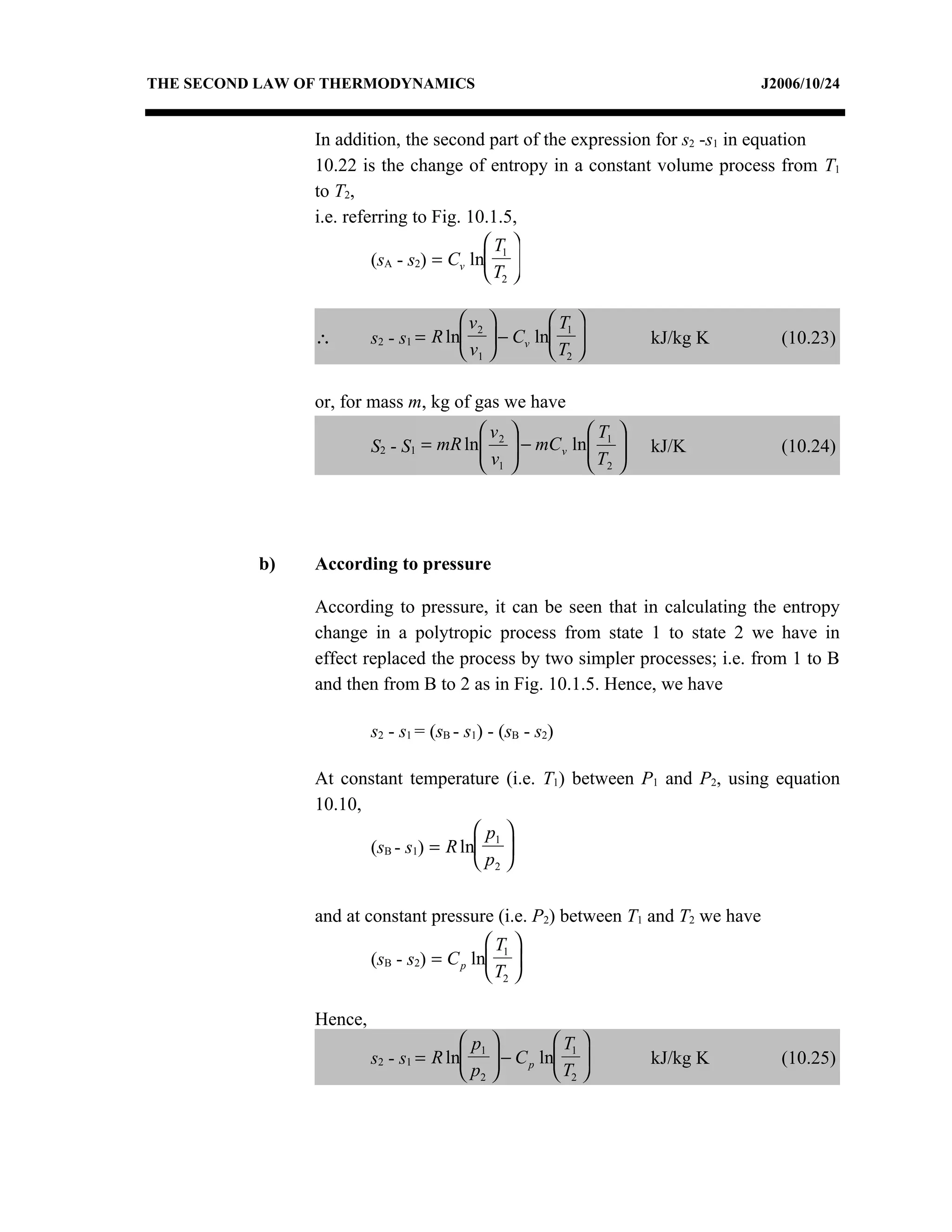
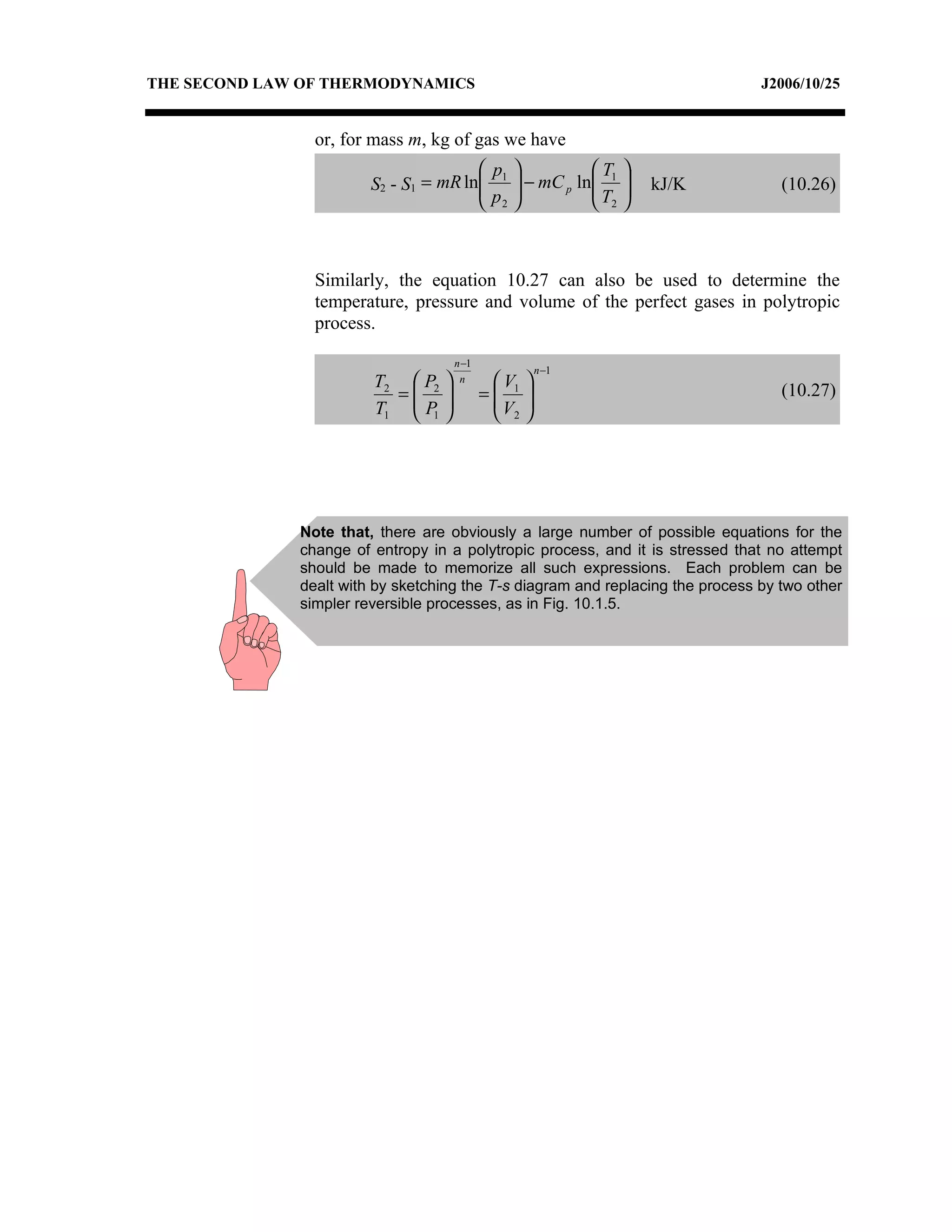
3) The relationships between pressure, volume, temperature and entropy for perfect gases during various reversible thermodynamic processes.