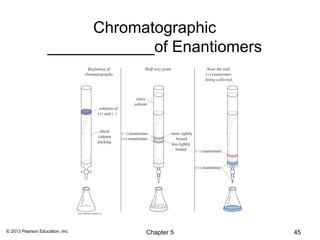
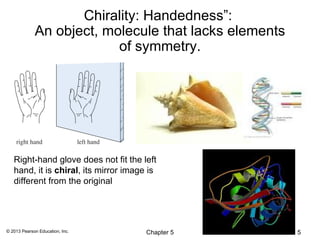
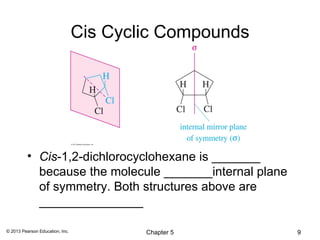
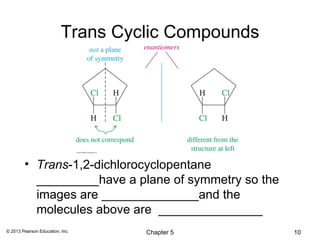
This chapter discusses stereochemistry and isomers. It defines several key stereochemistry terms:
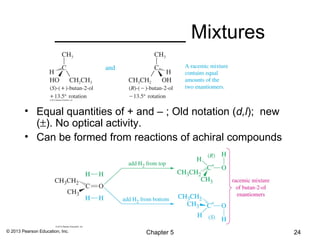
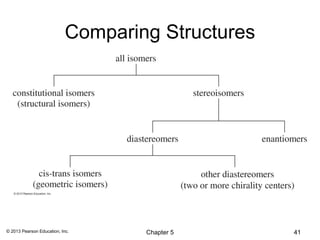
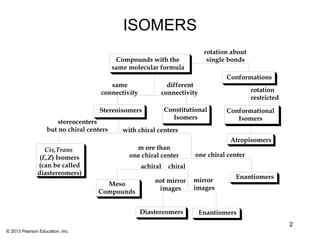
1) Stereoisomers as compounds with the same molecular formula but different connectivity or arrangements of atoms in space. The main types are constitutional isomers, conformational isomers, and stereoisomers.
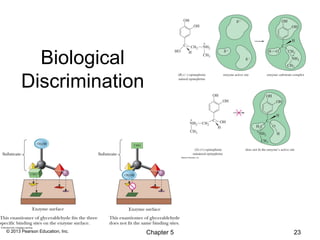
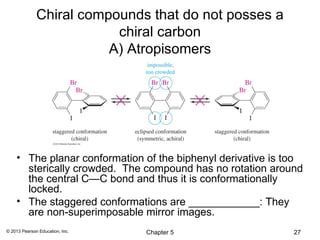
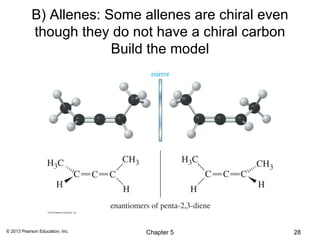
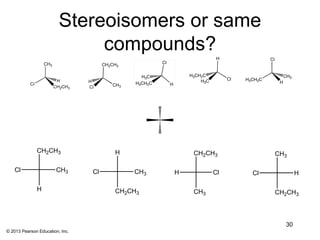
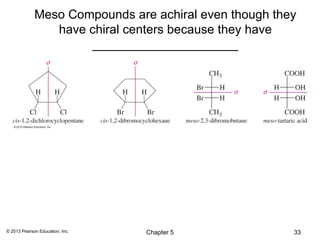
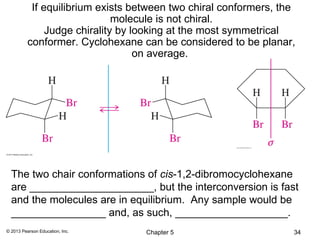
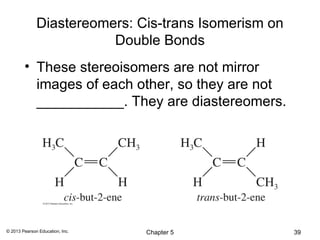
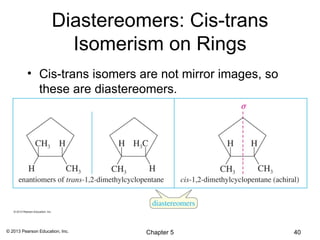
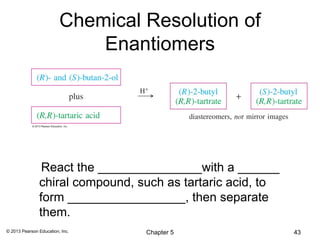
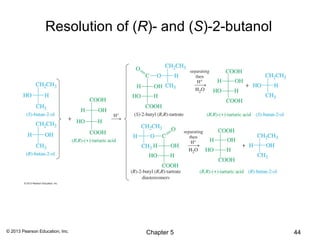
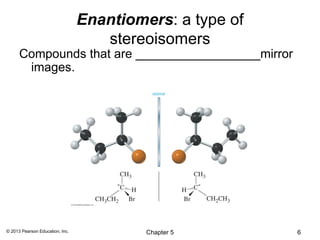
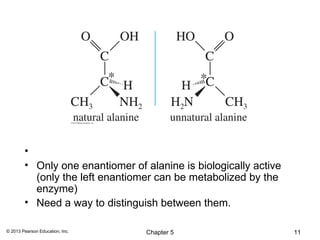
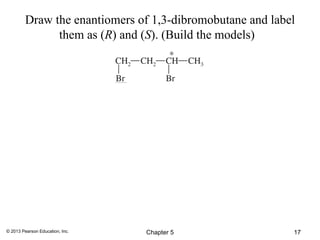
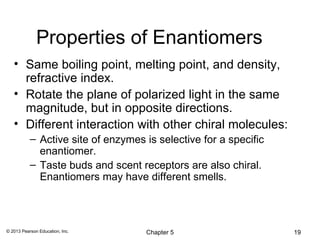
2) Stereoisomers are further divided into enantiomers, which are non-superimposable mirror images of each other, and diastereomers, which are not.
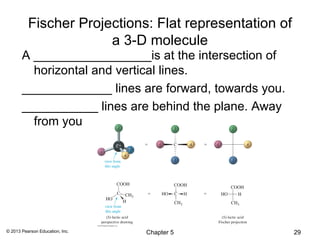
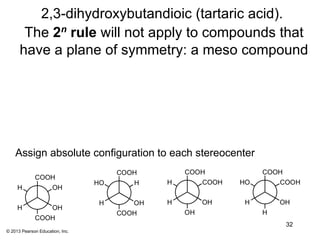
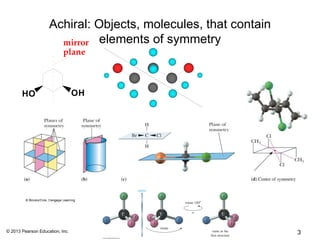
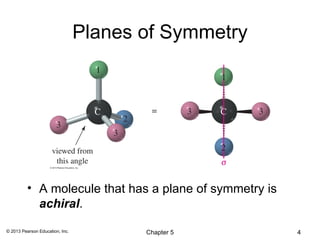
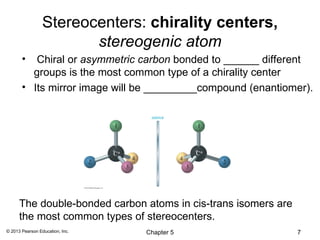
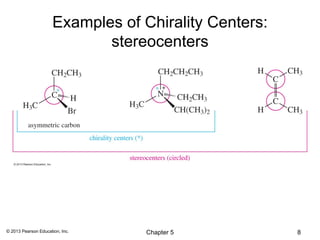
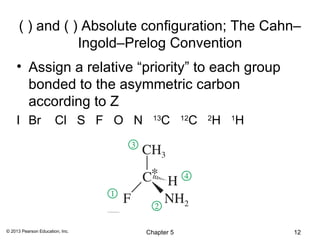
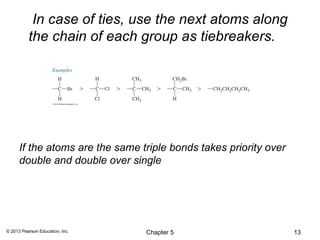
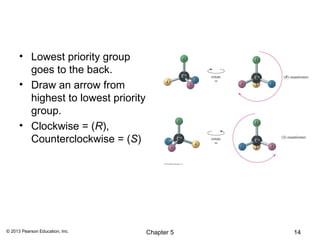
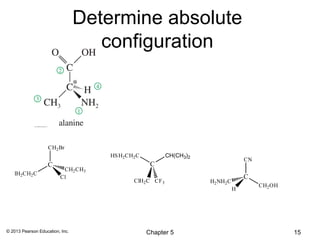
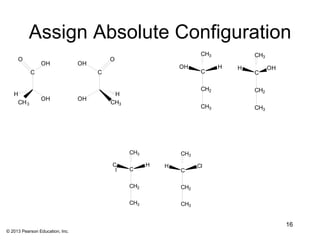
3) Chirality and stereocenters are discussed as the basis for stereoisomerism. The Cahn-Ingold-Prelog rules are introduced for assigning configuration to stereocenters.

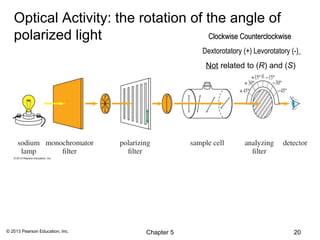
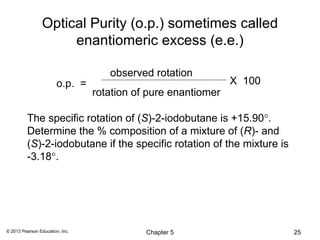
![Specific Rotation
Observed rotation depends on temperature, wavelength of
light, _________________________________________
Normalize:
[α] = α (observed)
c•l
α(observed) is the rotation observed in the polarimeter
c is concentration in g/mL,
l is length of sample cell in decimeters.
© 2013 Pearson Education, Inc. Chapter 5 21](https://image.slidesharecdn.com/ch5stereo-120624024546-phpapp02/85/Ch506-25-21-320.jpg)