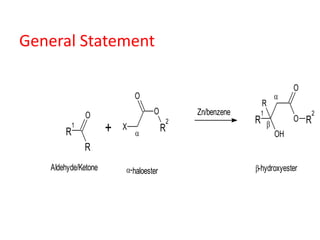
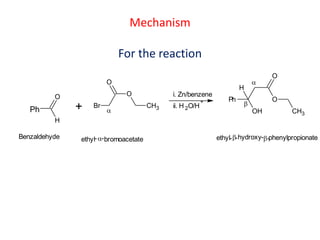
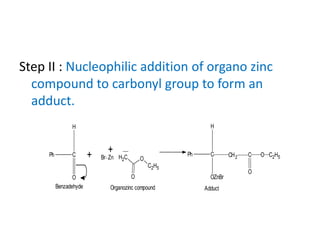
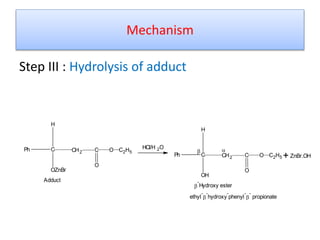
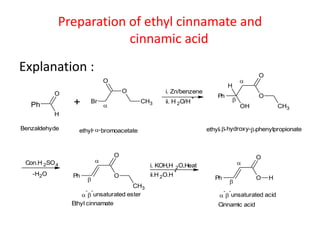
The document discusses the Reformatskii reaction, where aldehydes and ketones react with α-haloesters in the presence of zinc in inert solvents to produce β-hydroxyesters. It outlines the mechanism of the reaction in three steps: formation of an organozinc compound, nucleophilic addition to a carbonyl group, and hydrolysis of the adduct. The applications include preparation of β-hydroxy esters, β-hydroxy acids, and α,β-unsaturated esters and acids.